the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Quantification of 21 sugars in tropospheric particulate matter by ultra-high-performance liquid chromatography tandem mass spectrometry
Pauline Bros
Sophie Darfeuil
Véronique Jacob
Rhabira Elazzouzi
Dielleza Tusha
Tristan Rousseau
Julian Weng
Patrik Winiger
Imad El Haddad
Christoph Hueglin
Gaëlle Uzu
Jean-Luc Jaffrezo
Sugars compose an important class of compounds by mass in atmospheric particulate matter (PM), often with biogenic and anthropogenic sources, many of them still poorly characterized. These sugars are mainly analysed by gas-chromatography coupled to mass spectrometry (GC-MS) or ion chromatography coupled with pulsed amperometric detection (IC-PAD). However, these techniques present several disadvantages such as a complex preparation for GC-MS, or a limited range of possible analytes and elevated limits of quantification for IC-PAD. This hinders our capability to perform analyses of extensive time series, in order to develop our knowledge of the phenomenology of these species. In this paper, we present the validation of an ultra-high-performance liquid chromatography tandem mass spectrometry (UHPLC-MS/MS) method for the simultaneous quantification of 21 sugars in atmospheric PM. The sample preparation is simple, fast and safe, consisting of an aqueous extraction. The method was validated in terms of linearity, accuracy, repeatability, reproducibility, and recovery. This technique showed excellent linearity (r2>0.99), precision (relative standard deviation RSD <25 %) and extraction yields (results between 70 % and 120 %). The suitability of the method for analyses of samples from sites with very low PM concentrations was demonstrated with samples from the High-Altitude Research Station Jungfraujoch (JFJ), Switzerland. A series of samples spanning a 6-year period is presented. Results for arabitol, levoglucosan and 2-methyl-tetrols display strong seasonality, due to seasonal variation in chemical production and boundary layer dynamics, with atmospheric convection and transport from the valleys to high altitudes mostly in summer. This simple and fast method facilitates the analysis of large sets of PM samples and sugar compounds, and opens the door to a better understanding and attribution of their sources.
- Article
(620 KB) - Full-text XML
-
Supplement
(940 KB) - BibTeX
- EndNote
Particulate matter (PM) is involved in many aspects of Human environments, including large impacts on climate change, health, dispersion of pollutants, or damages to all compartments of the critical zone. Some impacts can be positive (the fertilization of Oceans), but most are negative, particularly when it comes to anthropogenic emissions. Assessing these impacts requires a proper understanding of some properties of PM (mass, size distribution, chemistry, physical and optical properties, etc.), and also a good parametrization of the fluxes in and out of the atmosphere, in order to develop Chemical Transport Models (CTM). CTM are a prerequisite for the global assessment of the impacts of the PM in Air Quality, Human health, or climate. However, the main component by mass of PM, the organic matter (OM), is still poorly understood, even after decades of research on its chemical composition, emissions and transformation processes, and observations worldwide of its variability. CTM are generally off by up to an order of magnitude, compared to measurements (Ciarelli et al., 2016), meaning that sources and/or processes of OM components are not fully captured. Indeed, the current state of the art concerning molecular characterization of OM cannot allow a better mass apportionment than about 25 % to 50 % at best of the total OM mass (Michoud et al., 2021; Wang et al., 2020). Obviously, several OM sources or formation processes in situ are still not understood nor their emission fluxes properly quantified.
While the formation of Secondary Organic Aerosol (SOA) is largely studied by a score of papers every year, the Primary Biogenic Organic Aerosol (PBOA) is largely disregarded, while studies point out its large contribution to the OM fraction (up to 25 %–35 % of the total OM mass on seasonal average for France) (Samaké et al., 2019). As opposed to Secondary Biogenic Organic Aerosol (SBOA), that is formed in the atmosphere from the biogenic volatile organic compounds (VOCs) emitted by biota, PBOA is a subset of organic PM that comprises all particulate material of biogenic origin directly entering the atmosphere. PBOA includes components like plant debris, fungi, pollen, and viruses (Amato et al., 2017; Elbert et al., 2007; Hummel et al., 2015). The current estimate of the global PBOA flux to the atmosphere is between 10 and 310 Tg per year (Hummel et al., 2015), compared to values of 21 (MACCCity) or 30 (CMIP6) Tg per year for particulate organic components from anthropogenic origins (Rémy et al., 2019). The wide uncertainty on the flux of PBOA indicates by itself the extent of our poor knowledge on this fraction. Moreover, PBOA is currently very rarely included in CTM's (Rémy et al., 2019). One attempt to model PBOA on the European scale using the COSMO-ART CTM (Hummel et al., 2015) showed that PM mass associated with solely fungal spore emissions could represent on yearly average 15 % of the total PM (about 25 %–30 % of organic PM) mass over Europe. A couple of papers very recently described the modelling of PBOA from fungal spore emission in CHIMERE (Vida et al., 2024) and EMEP models (Felix-Lange et al., in progress). A better assessment of this PBOA fraction is all the more important, since they also have a significant impact on the oxidative potential (OP) of PM, which can be a prevalent source in OP contribution at spring (Samake et al., 2017; Dominutti et al., 2023).
A large part of the PBOA fraction is related to the fungi emission sources (Hummel et al., 2015; Marynowski et al., 2020; Wang et al., 2020). Therefore, the seasonal cycles of some sugars and sugar alcohols (S and SA) concentrations observed in atmospheric PM can be directly related to the microbiology of large biomes. However, this fungi-sugar link has only been documented for a limited number of sugars (e.g. arabitol, mannitol) and cases (Marynowski et al., 2020; Samaké et al., 2020), and the main sources and drivers of many other S and SA in PM have not been identified yet. Currently, the mechanisms of S and SA emissions (along with their full organic cortege) to the atmosphere, and their fate and transfer to other ecosystem compartments remain poorly understood. Anhydrosugars, another form of sugars in atmospheric PM can be natural but also anthropogenic, such as levoglucosan and its isomers mannosan and galactosan, which are commonly used as a tracer for the assessment of PM from biomass burning (natural wildfires, domestic wood heating or cooking). These anhydrosugars – carbohydrate derivatives formed by the elimination of a water molecule – have been of primary importance for source apportionment studies since at least 15 years (Herich et al., 2014; Weber et al., 2019), and are now a basic measurement for PM analysis. The main suspected sources of other sugars measured in atmospheric PM are summarized in Table S1. Many of the links between chemical species and sources proposed in the literature Table S1 are still tentative, with only few comprehensive measurements for many of the species cited.
Analyses of sugars are mostly performed by gas chromatography coupled with mass spectrometry (GC-MS) (Vincenti et al., 2022). This method, with high selectivity and specificity, is generally performed with large sample volume and a sample preparation using derivatization of the hydroxyl groups (Graham et al., 2002). The introduction of ion chromatography coupled with pulsed amperometric detection (IC-PAD) in the mid 2000's (Engling et al., 2006; Yttri et al., 2007) made the determination of sugars in PM easier, at the expense of a narrowing of the range of the accessible sugars due to potential interferences with lack of column separation. Nevertheless, IC-PAD allows for the determination of 8–12 S and SA without too many interferences, including the three anhydrosugars (AS) (levoglucosan, mannosan, galactosan) (e.g., Oduber et al., 2021). In recent years, liquid chromatography tandem mass spectrometry (LC-MS/MS) has become more common in laboratories due to its greater efficiency and resolution, speed, simplicity, and low cost compared to more conventional chromatographic techniques (López-Ruiz et al., 2019). Indeed, LC-MS/MS can offer a much larger chemical speciation of S and SA and much lower limits of quantification than IC-PAD, together with much simpler sample preparation than GC-MS.
The analysis of sugars by LC-MS/MS is not a new topic, as they have been quantified for several years in different fields including food, plant and forensic fields. Indeed, fructose, glucose and sucrose are widely analysed in plant and food using such methods: in plants such as arabidopsis and rice (Ito et al., 2014), potatoes and strawberry (Georgelis et al., 2018), grapes (Gika et al., 2012), in bread (Nielsen et al., 2006), fruit juices (Rego et al., 2018), honeydew (Nguyen et al., 2020) and fermentation processes (Pismennõi et al., 2021). In a completely different field, a LC-MS/MS method was validated to analyse eleven sugars and sugar alcohols related to explosive (Tsai et al., 2022). The most comprehensive methods reported to date allow the simultaneous analysis of no more than 19 sugar compounds (Gika et al., 2012; Ito et al., 2014). Even if application areas were numerous, the implemented LC-MS methods were essentially the same. Indeed, the LC separation was performed in an HILIC (Hydrophilic Interaction Liquid Chromatography) mode with column coated with amine or polyamide groups in order to enhance the polar compound retention and selectivity. Moreover, the MS was operated in negative mode and mostly using multiple reaction monitoring (MRM). In summary, these runs exhibit certain similarities with our intended approach, but remain constrained with respect to the range of analytes quantified and are most likely restricted to substantially higher concentration levels than expected for atmospheric PM samples, though probably impacted by more complex matrices. These observations collectively indicate that our experimental design is feasible; however, its implementation require substantial methodological refinement and further development to extend the analytical scope, particularly to encompass a broader set of species including numerous isomeric forms.
This work was focused on the validation of a UHPLC-MS/MS method for the simultaneous quantification of 21 sugars in PM, with a very simple and fast sample preparation. It presents the overall analytical method and its performances, including limits of quantification, linearity, intermediate precision studies, accuracy, and recovery. As a proof of concept, applicability, and to showcase challenging real-world samples, a timeseries of measurements of some species are presented for samples from Jungfraujoch (JFJ) site, Switzerland (3580 m a.s.l.), an alpine high-altitude international research station (Bukowiecki et al., 2016). Ultimately, the high sensitivity and ease of this method could pave the way for numerous additional studies on these families of chemical species, enhancing our understanding of atmospheric sugar cycles and their connection to biological processes.
2.1 Standards and reagents
A large literature survey was conducted to make an assessment of the sugars, sugar alcohols and anhydrosugars that have previously been cited in the literature concerning atmospheric particulate matter, as synthetized in Table S1 in the Supplement. A large selection of compounds was initially tested during the method development and 28 of them were kept for the development processes and method validation. Finally, only 21 of them were properly quantified and monitored for routine analysis. The chemical structures and physicochemical properties of these target compounds are shown in Table S2 and their suppliers are presented below.
Adonitol (BioXtra, ≥99.0 % (HPLC), L-(+)-Arabinose (BioUltra, ≥99.5 % (sum of enantiomers, HPLC)), L-(−)-Arabitol (≥98 % (GC)), Erythritol (CRM), L-(+)-Erythrulose (≥85 % HPLC), D-(−)-fructose (≥99 % (HPLC)), D-(+)-galactose (≥99 % (HPLC)), D-(+)-Glucose (BioUltra, anhydrous, ≥99.5 % (sum of enantiomers, HPLC)), Glycerol (≥99.5 %), Inositol (CRM), D-Lactose monohydrate (BioUltra, ≥99.5 %), Levoglucosan (1,6-Anhydro-β-D-glucose (99 %)), Maltitol (≥98 % (HPLC)), D-(+)-Maltose monohydrate (BioUltra, ≥99.0 %), D-Mannitol (BioUltra, ≥99.0 % (sum of enantiomers, HPLC)), D-(+)-Mannose (synthetic, ≥99 % (GC)), D-(+)-melezitose hydrate (≥97 % (HPLC)), L-Rhamnose monohydrate (≥99 %), D-(−)-Ribose (≥99 % (GC)), Sedoheptulosan, D-sorbitol (BioUltra, ≥99.0 % (HPLC)), Sucrose (Saccharose ≥99.5 % (GC), BioXtra), D-Threitol (99 %), D-(+)-Trehalose dihydrate (CRM), D-(+)-Xylose (≥99 % (GC)) and D-(+)-Xylose (≥99 % (GC)) were obtained from Merck-Sigma (France). Galactosan (1,6-Anhydro-beta-d-galactopyranose 97 %) and Mannosan (1,6-Anhydro-beta-d-mannopyranose 97 %) were purchased from Combi-Blocks (USA). 2-methyl-D-Erythritol (2S, 3R) and its 3 isomers (2-methyl-L-Erythritol (2R, 3S); 2-methyl-D-treitol (2S, 3S) and 2-methyl-L-treitol (2R, 3R)) were synthesized by Plateau Synthese Organique, Département de Chimie Moléculaire (University of Grenoble Alpes, France) according to Ghosh et al. (2012). Isotopically labelled standards, myo-Inositol (1,2,3,4,5,6-D6, 98 %), D-Glucose (1,2,3,4,5,6,6-D7, 97 %–98 %) and 1,6-Anhydro-beta-d-glucose (Levoglucosan) (U-13C6, 98 %) were obtained from Eurisotop. Ammonia solution (25 %, LC-MS grade), Water (Rotisolv®, LC-MS grade) and Acetonitrile (Rotisolv® ≥99.95 %, LC-MS grade) were obtained from Roth.
2.2 Calibrators preparation
Individual stock solution of each target analyte and each internal standard are prepared in MilliQ® water (18.2 MΩ cm) at 1000 ppm and stored at 4 °C. A stock solution of all target analytes (MIX-28) is prepared by mixing each target analytes in MilliQ® water. In parallel, a stock solution of the three internal standards, inositol-D6, glucose-D7 and levoglucosan-13C6 (MIX-IS) is prepared in the same manner at 10 ppm. MIX-28 and MIX-IS water solutions are used to prepare an eight-point calibration curve in solvent with final concentrations of 90 % acetonitrile, 0.005 % NH4OH and 8 ppb for internal standards. Calibration range for each target analyte was adapted from a wide variety of analysed real samples, and is displayed in Table 2.
2.3 UHPLC-MS/MS conditions
All measurements were performed with ultra-high-performance liquid chromatography (ExionLC – AD binary pump, Shimadzu) coupled to a tandem mass spectrometer (AB SCIEX 5500 QTRAP). The column oven was set at 30 °C. The injection volume was 30 µL. The chromatographic separation was performed on a Luna Omega Sugar (150 mm × 2.1 mm × 3 µm) column from Phenomenex (France) with a compatible guard column (SecurityGuard Cartridges, Sugar, 4 × 2.0 mm ID, Phenomenex). This column is of hydrophilic interaction chromatography (HILIC) type and is adapted to hydrophilic interaction and separation of hydrophilic compounds such as sugars, in aqueous matrix. One overall goal of this development was to get simplified water extraction of the samples, but it made direct injection impossible since HILIC mode supports only comparable sample and eluants initial conditions (10 % water, 90 % acetonitrile). Other columns with such specifications (BEH Amide 100 mm × 2.1 mm × 1.7 µm from Waters, and HPLC Ultra Amino 150 mm × 3 mm × 3 µm from Restek) were tested but did not provide such good results in terms of separation (peak shapes, return to baseline) after optimization, compared to the Luna Omega Sugar column.
Mobile phase (A) was 0.002 % ammonia in H2O (LC-MS grade) and mobile phase (B) was pure acetonitrile (LC-MS grade). The gradient elution was programmed as follows (2 isocratic steps followed by the rinsing of the column and equilibration): 0.00–9.00 min, 10 % mobile phase (A), 0.45 mL min−1; 10.00–23.00 min, 20 % mobile phase (A), 0.40 mL min−1; 23.01–28.00 min, 40 % mobile phase (A), 0.45 mL min−1; 28.01–29.01 min, 10 % mobile phase A, 0.45 mL min−1; 29.01–36.00 min, 10 % mobile phase A, 0.45 mL min−1. Total run time was 36 min. In order to limit soiling of the MS system, the divert valve was programmed to MS analysis only between 1 and 25 min. The autosampler temperature was set to 5 °C in order to limit potential evolution of the samples during the analytical batch.
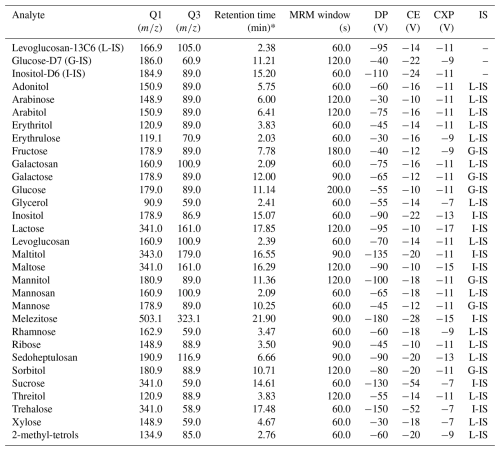
The mass spectrometer was operated in negative electrospray ionization (ESI) mode using a scheduled multiple reaction monitoring (MRM). The ESI parameters were optimised and finally set to the following ones: spray voltage −4500 V, source temperature 400 °C, Ion Source Gas 1 (GS1) 40.0 psi, Ion Source Gas 2 (GS2) 60.0 psi. Q1 and Q3 mass resolution is ±0.1 Da. The MRM transition per compounds were determined by infusion of individual standards at 100 ppb in 10/90 % water/acetonitrile, in presence of 0.005 % NH4OH. For each target analyte, infusion of monospecific standard solution was made: a full scan mass spectrum was acquired to determine its precursor ion. Then, product ions scans were acquired on the selected precursor ion to select a product ion of interest. One MRM transition is chosen to characterize each compound. All MRM parameters for each analyte are summarized in Table 1.
Given the fact that some analytes are isomers, the identification of each analyte is also performed using its elution order and its retention time. Given the nature of the HILIC phase, retention time may slightly change between each batch, and a standard solution is used to recalibrate the retention time of each analyte before batch launches. The system is controlled with the Analyst® software version 1.7 (AB SCIEX). All data processing (integration peak and quantification) was conducted on SCIEX OS v1.7, using individual internal standards for each compound with the external calibration to correct potential ionisation variations in the source.
2.4 Sample collection and preparation
Ambient aerosol samples were collected at the High Altitude Research Station Jungfraujoch (JFJ) (3580 m a.s.l., Switzerland) as part of the Swiss National Air Pollution Monitoring Network (NABEL). The JFJ station is located in the free troposphere with regular intrusion of planetary boundary layer air masses. It generally offers very low background aerosol concentrations. Samples were collected over 24 h on quartz fiber filters (150 mm diameter discs, Pallflex 2500 QAT-UP) using DHA-80 high volume samplers (DIGITEL) with a PM10 inlet, operating at 45 m3 h−1. After sampling, the filter samples are transported back to the laboratory for gravimetric determination of the PM10 mass concentration. Subsequently, the filters are put inside a glassine envelope (PAWI Packaging Schweiz AG), sealed in polyethylene bags and stored at −18 °C until further analysis (Hueglin et al., 2005). The samples studied here were composites of 4 daily filters distributed over 2 weeks, for a period of several years from 2011–2016. A total of 136 composite samples and 11 field blanks were analysed.
For the extraction of analytes of interest, a portion of the quartz filter (typically a total of 10.2 cm2 for each composite sample from the JFJ site) was used. The filter was extracted in 6 mL of MilliQ® water with vortex shaking for 20 min. This sample preparation was adjusted for JFJ samples for which very low concentrations of sugars were expected. In cases of daily samples from most low altitude sites, 5 cm2 of quartz filter are generally extracted in 7 mL of water (Aas et al., 2025). Then, the supernatant is filtered with 0.2 µm Ion Chromatography Acrodisc® 13 pre-washed with ultrapure water. If necessary, extracts are stored in a freezer at −20 °C for later analysis. Finally, 100 µL of the extract is diluted with 900 µL of the dilution mixture (99 % acetonitrile, 0.005 % NH4OH and internal standard adjusted at 9 ppb from MIX-IS) to reach chromatographic equilibration conditions, and then analysed by UHPLC-MS/MS. Samples stay 69 h at most in the refrigerated autosampler before analysis, in order to avoid potential aging processes.
2.5 Method performance evaluation
2.5.1 Method validation
The performance of the method was evaluated by studying linearity, limit of quantification (LOQ), limit of detection (LOD), precision, bias, and recovery. The linearity was evaluated with 8 points of calibration per compound. The calibration curves were determined using least-squares linear regressions. The LOD was determined as the minimal detected standard with a signal/noise ratio larger than 3. Precision studies including intra-day and inter-day evaluations, and bias were determined with the injection of a standard every 8 injections during each batch. Accuracy was evaluated for levoglucosan by comparison to standard reference material NIST® SRM® 2786 (Fine Atmospheric Particulate Matter). Recovery was evaluated on summer and winter filters collected on the roof of the laboratory in Grenoble (France), to test for a potential seasonal effect.
2.5.2 Calculation and analytical batch
The quantification is based on the isotope dilution with the addition of isotope labelled sugars (inositol-D6, glucose-D7 and levoglucosan-13C6) into the injected sample to correct analytical variation of the source of the mass spectrometer. Calibration curves are drawn by plotting the area ratio (Compound area/IS area) against the concentration ratio (Compound concentration/IS concentration). Thus, the sample concentration, in ppb, can be determined with the area ratio of the sample. Finally, results were converted in ng m−3 using the extracted surface of filter, the volume of air collected, the initial volume of water for the extraction, the dilution factor with the eluant, and taking into account the average of the sample field blanks of the corresponding field campaign.
In daily-routine, full set of calibration solutions are injected every 40 samples (about every 24 h of analysis), and the standard 3 of the calibration range (STD3) is injected every 8 injections as a quality control. These repetitions are performed to ensure that the LC conditions, in particular column ageing, are compatible with an accurate quantification of samples.
3.1 Sample preparation
The sample preparation exclusively in water and without derivatization is intended to be simple, limiting the preparation stages, the handling, the possibilities of contamination, and the use of solvents and other chemicals. All of these convey major advantages for this preparation in terms of time consumption, consumables costs, and required manpower. Moreover, these same aqueous extracts can be directly used for other analyses such as major ions and organic acids by ion chromatography coupled to mass spectrometer on anion canal (IC-MS) (Glojek et al., 2024), sugars analysis by IC-PAD (Samaké et al., 2019), or HUmic LIke Substances analysis using a TOC analyser after separation by HPLC-Fluorescent (Baduel et al., 2009). Therefore, these extracts, the associated costs and the consumption of the sample filter surface can be shared between these different analyses, which is another big advantage.
3.2 Method validation
3.2.1 Retention times and co-elutions
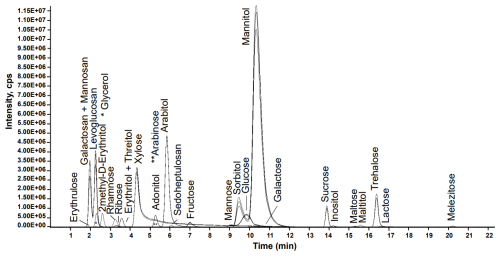
The 28 compounds eluated along the 25.0 min MS analysis period (Fig. 1) and retention times were repeatable with a relative standard deviation (RSD) below 2.0 % for all compounds for 146 successive injections over a period of 61 hours. However, some chemical species cannot be separated in this run.
First, the 2-methyl-erythritol and 2-methyl-threitol four stereoisomers are co-eluted and present identical MRM transition, but they also provide the same response factor. 2-methyl-D-Erythritol is then used as the quantification standard and represents therefore the four isomers, named commonly as 2-methyl-tetrols. Second, the two compounds in each pair galactosan/mannosan and erythritol/threitol present the same retention time and the same MRM transitions, but with different response factors in terms of intensity for identical concentrations. Their quantification cannot be achieved with this method.
Finally, the Luna Omega Sugar column was globally suitable for the analysis of the other sugar compounds from Table 1, but it must be replaced around every 300–600 injections due to the column ageing. Indeed, a drift of retention time was observed with ageing, with (for the example of Fig. S1 in the Supplement) an average over all 28 compounds of 0.5 min after 512 injections, which can go up to 2.2 min for the case of melezitose at the end of the run (Fig. S1). This ageing may be due to anion (such as Cl− from extract samples) progressive occupation of active separation sites (like amine), leading to less effective separation of sugars and retention time decrease through numerous and charged sample injections (personal communication J. Lacouchie-Payen, Phenomenex, 2022). The pairs adonitol/arabitol and sorbitol/mannitol tend to co-eluate with increased column ageing (Fig. S1). This cannot be reversed with any cleaning procedure. A criterion to discard an aged column is the difficulty to separate completely those 2 pairs of compounds.
3.2.2 Cases of tested but not analyzable compounds
Several compounds were tested and monitored but no result can be returned. This is the case for arabinose and galactose which were not automatically detected in most of the analytical batches due to their weak intensity and their elution at the end of the trailing peak of xylose and glucose, respectively. Therefore, linearity cannot be evaluated, neither proper quantification. However, our experience with many series of samples in various atmospheric conditions is that they are not present in the atmospheric PM10 with the LOD mentioned in Table 2.
Finally, glycerol is also a specific case. It has been reported in plants in high concentrations (Gerber et al., 1988) and has an influence on plant flowering (Lazare et al., 2019). Moreover, Kang et al. (2018) found higher level of glycerol in PM2.5 filter samples during winter and autumn when vegetation decays and fungal population increases. Several studies worldwide confirmed the presence of glycerol in PM and associated it with biomass burning (Zangrando et al., 2016). Our results of the method performance evaluation showed that glycerol can be easily contaminated. This can take place at any time from the sampling to the LC-MS/MS analysis, as currently glycerol is present in everyday life (Bagnato et al., 2017) as it is included in a large variety of products such as cosmetics, food (E422, (EFSA Panel on Food Additives and Flavourings (FAF) et al., 2022), pharmaceutical and tobacco products in particular e-cigarettes (Kubica, 2023). We choose not to report glycerol concentrations since results obtained are often (but not always) erratic. More work must be done for understanding the contamination processes, limiting them, and obtain trustable results.
3.2.3 Linearity and Limit of Detection (LOD)
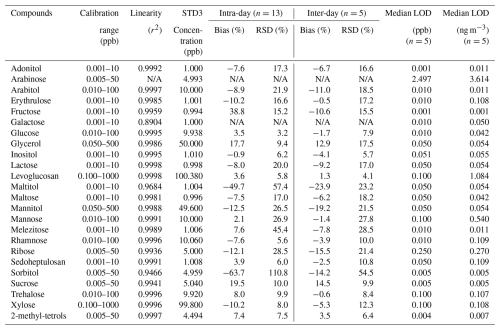
The calibration range defined for this method was determined for each compound in order to represent the full range of the concentrations generally expected from atmospheric samples prepared with our usual sampling and extraction conditions. This was determined following our experience for the compounds measured from a large literature review. The linearity was evaluated for the full 8 points calibration range of each compound. A very good correlation between prescribed and observed concentrations were observed with r2 above 0.99 (Pearson's criteria), except for maltitol and sorbitol (Table 2).
The analytical Limit of Detection (LOD) is the smallest standard detected in the analytical run, with a signal/noise ratio >3. Values are displayed in ppb in the extract. The analytical LOD is compound-dependent and varies from 0.001–2.5 ppb, and after conversion in ng m−3 using our standard sampling and extraction conditions, from 0.001–3.6 ng m−3 (Table 2). However, the actual field LOD will vary according to the field blank value, for each compound and each field campaign.
3.2.4 Intermediate precision studies
In our classical analytical batch, the standard 3 (STD3) is injected every 8 injections as a quality control. Bias is calculated for STD3 as follows (Eq. 1):
94 % of intra- and inter-day bias results obtained over a period of 5 d were below a 25 % bias, meaning that the method quantification is robust (Table 2). 83 % of intra- and inter-day RSD results obtained over a period of 5 d were also below 25 %, meaning that this UHPLC-MS/MS method is repeatable and reproducible (Table 2). We note that for intra-day, more RSD results (n=6) were larger than 25 %, compared to inter-day RSD results (n=3). However, for the compounds with particular interest (like the ones used in source apportionment studies such as arabitol, fructose, glucose, inositol, levoglucosan, mannitol, sucrose, trehalose and 2-methyl-tetrols), RSD results were similar between intra-, and inter- day. Conversely, the species with the worst results happen to be of lower interest for atmospheric samples, with maltitol never being detected with a LOD of 0.05 ng m−3, and sorbitol rarely seen with a LOD of 0.005 ng m−3.
3.2.5 Accuracy
Accuracy was evaluated for levoglucosan by aqueous extraction and UHPLC-MS/MS analysis of fine particulate matter reference material of Standard Reference Material (SRM®) 2786 from the National Institute of Standards and Technology (NIST®) (NIST® SRM® 2786) (n=6). Precision was 4.2 % and bias was 10.0 % which were compliant with RSD <15 % and bias ±15 %. In this SRM, concentrations for galactosan and mannosan are also available, but due to coelution and identical MRM transitions with different response factors in terms of intensity for identical concentration, their quantification is not guaranteed with this method. Currently, to our knowledge, no SRM is available for the other sugars monitored in this UHPLC-MS/MS method. However, for information purposes only, results of the concentrations (mg kg−1) for 21 sugars of the NIST® SRM® 2786 are provided in Table S3.
3.2.6 Recovery
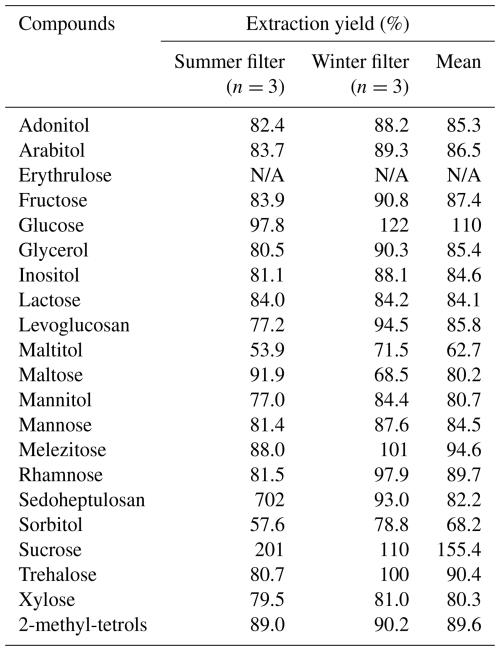
Extraction yield was assessed by comparing results from samples spiked at the STD3 level before and after extraction. The experiment was performed on summer (n=3) and winter filters (n=3) collected in the urban background atmosphere of Grenoble. The average chemical composition of the PM10 for a large range of compounds is described in Borlaza et al. (2021). It should be noted that several of the compounds presented in Table 3 present very low concentrations (sub-ng m−3 or lower), explaining some of the very high results (like sedoheptulosan). However, mean extraction yields were satisfying with most of the results between 70 % and 120 % (Table 3).
In summary, this UHPLC-MS/MS method provides reliable and low-level quantification for 21 sugars for atmospheric fine particles including sugars (erythrulose, fructose, glucose, lactose, maltose, mannose, melezitose, rhamnose, ribose, sucrose, trehalose, xylose), sugar alcohols (adonitol, arabitol, inositol, maltitol, mannitol, sorbitol, 2-methyl-tetrols) and anhydro-sugars (levoglucosan, sedoheptulosan). New sugars (adonitol, erythrulose, fructose, maltitol, maltose, melezitose, sedoheptulosan, sucrose, trehalose, xylose and 2-methyl-tetrols) not quantified with traditional IC-PAD method (Engling et al., 2006; Yttri et al., 2007; Samaké et al., 2019) are provided and will allow further exploration of their sources and the processes they undergo in the atmosphere.
3.3 Jungfraujoch 6-year time series of samples
3.3.1 Atmospheric concentration calculations
The interest of this UHPLC-MS/MS method is demonstrated with the analysis of a long time series of samples collected in the low concentration levels environment of the High Altitude Research Station Jungfraujoch (Switzerland). These samples can be regarded as challenging, insofar as they usually contain very low PM values and hence sugar concentrations. With the simple and fast sample preparation method, the 136 composite samples and 11 fields blanks were prepared in two days. After analysis, the results of composites and field blanks were processed in the same manner in SCIEX OS. The field limit of quantification (Field LOQ) was calculated for each compound (Table S4), based on the field blanks results, as follows: (Eq. 2)
When results for composite samples were above this Field LOQ, the value of mean field blank was subtracted from the initial sample value in ppb, subsequently converted in ng m−3.
3.3.2 Evolution of the concentrations
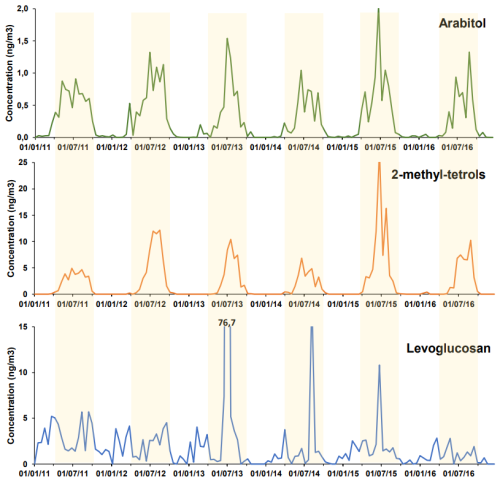
Median and seasonal concentrations of the 21 quantifiable sugars are presented in Table S4. The time series of 3 sugars of interest (arabitol, 2-methyl-tetrols and levoglucosan) are plotted on Figure 2 over a 6-year period, with a time resolution of 20 composite samples per year.
Arabitol displays values between LOD (0.011 ng m−3) and 2 ng m−3, with seasonal variation patterns (May–November occurrence) and maximal values in high summer. Interannual variability of the maxima summer concentrations are between 0.6 and 2 ng m−3. Following the same pattern as arabitol, the concentrations of other primary biogenic (PBOA) sugars such as mannitol, glucose, fructose, trehalose, and melezitose also display strong seasonal variations with much larger concentration in summer that are below LOD in winter. All concentrations (Table S4) are much lower (by a factor of about 20) than usual concentrations in low-altitude environments in France, and also in Alpine valleys (e.g. in Grenoble (Samaké et al., 2019) or Slovenian valleys (Glojek et al., 2024)).
In comparison, the secondary organic aerosol (SOA) tracer 2-methyl-tetrols, associated with the oxidation products from isoprene emissions, also displays strong seasonality with inter-annual variability of concentrations, but with a shorter occurrence of this compound compared to arabitol, restricted to June-to-early September (Fig. 2). Contrary to primary biogenic sugars, concentrations reach much higher values (between 5 and 25 ng m−3 for each summer maximum), and are only 4 to 12 times lower than in Alpine lower altitudes (Glojek et al., 2024).
Finally, the levoglucosan timeseries is really noisy, without any particular seasonal pattern (Fig. 2). The concentrations are low (<6 ng m−3), except for three specific episodes in July months, reaching up to 77 ng m−3. Those low background concentrations in summer are equivalent to those in lowlands, but in winter are 500–1000 times lower than winter concentrations in alpine valleys that can reach up to 8 µg m−3 in cases of temperature inversion layers in narrow Alpine valleys (Bonvalot et al., 2016; Herich et al., 2014).
These results align well with biogeochemical understanding in light of the specific environmental context of the JFJ site, where the atmospheric signal can only be captured when both production in the surrounding lowland areas and efficient transport to high altitudes occur concurrently. The levoglucosan time series is consistent with findings from a 14-months Aerosol Chemical Speciation Monitor (ACSM) study at JFJ (Fröhlich et al., 2015), which highlighted the absence of local planetary boundary layer (PBL) sources during summer, and the lack of vertical PBL transport to the site in winter, despite elevated winter PBL concentrations primarily driven by domestic wood burning. The few observed peaks are most likely associated with long-range transported biomass burning plumes, such as those originating from Canadian wildfires in late June and July 2013, which were confirmed to have reached JFJ based on Copernicus Atmosphere Monitoring Service (CAMS) data.
Similarly, the distinct seasonality observed for arabitol and 2-methyl-tetrols (representing PBOA and SOA, respectively) reflects the influence of local sources within the PBL combined with efficient upward transport to JFJ during summer, when convective mixing is favored by warmer conditions. In contrast, the absence of both significant sources and vertical transport during winter explains the minimal concentrations observed during this season. Furthermore, the observed concentration differences between arabitol and 2-methyl-tetrols may reflect variations in local PBL composition as well as differential chemical aging during transport to JFJ. The full time series of sugars concentrations will be discussed in a dedicated publication, including a larger array of chemical species.
This UHPLC-MS/MS method for molecular-resolution quantification of sugars in atmospheric PM10 samples offers several advantages. First, the sample preparation is intended to be simple, with an extraction in water, without any other steps like solvent reduction or derivatization. This conveys a major advantage of this preparation in terms of time consumption, consumables costs and required manpower. The preparation of the series of samples (n=147) taken as an example in this work was performed in only two days. Moreover, this sample preparation can be adjusted according to the type of samples, including low concentrations as in this work.
Analytical results were very good in terms of linearity (r2>0.99), precision (RSD <25 %), extraction yields (results between 70 % and 120 %). Accuracy was compliant by comparison with the NIST® SRM® 2786. This method offers a larger number of sugar compounds properly quantified than with traditional IC-PAD method), which will allow enhanced exploration of sources and fate of a larger array of sugars in the atmosphere.
This highly sensitive method was successfully applied to 136 composite samples and 11 field blanks from Jungfraujoch (JFJ), Switzerland, a high latitude alpine station. These JFJ results provide concentrations for the European free troposphere, and indicate large seasonal variations over a 6-year period for many compounds, including arabitol, 2-methyl-tetrols and levoglucosan.
The high-resolution molecular-level chemical speciation achieved with this approach enables to further explore sugars sources and processes. This method has already proven effective across a range of sites and sample types: PM10 samples in Alpine valley sites, like in Kanal (Slovenia) (Glojek et al., 2024) and Grenoble (France) (Cruaud et al., 2025), but also in cloud water (Bianco et al., 2025), snow samples (Schivalocchi et al., 2025) and ice cores samples (Piot et al., 2025). The wide applicability across matrix – from atmospheric aerosols to snow, cloud water, and ice cores – demonstrates its potential to greatly enhance our understanding of the dynamics of sugar compounds in various environmental compartments. As such, this method opens new opportunities for in-depth investigations into the sources, transformations, and transport of sugars in the atmosphere, as well as their interactions with climatic and biological processes.
No software or code available. The Sciex OS software for UHPLC-MS/MS quantification was acquired with our instrument in 2019, and used in a conventional manner.
Raw data for JFJ sugar concentrations are available on request to the corresponding author.
The supplement related to this article is available online at https://doi.org/10.5194/amt-18-6315-2025-supplement.
PB: Investigation, Writing – Original Draft, Visualization, Writing – Review & Editing; SD: Methodology, Investigation, Writing – Review & Editing, Visualization, Supervision; VJ: Methodology; RE: Resources, Data Curation; DT: Investigation; TR: Investigation; JW : Investigation, Writing – Review & Editing; PW: Resources, Writing – Review & Editing, Funding acquisition; IEH: Resources, Writing – Review & Editing, Funding acquisition; CH: Resources, Writing – Review & Editing, Data Curation; GU: Funding acquisition; J-LJ: Conceptualisation, Writing – Review & Editing, Supervision, Funding acquisition, Project administration.
The contact author has declared that none of the authors has any competing interests.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors. Views expressed in the text are those of the authors and do not necessarily reflect the views of the publisher.
We are thankful to Pierre-Yves Chavant and Mathieu Curtil from Plateau Synthèse Organique at Département de Chimie Moléculaire of the University of Grenoble Alpes (France) for the synthesis of 2methyl-D-Erythritol and its isomers.
Sample collection was carried out by the Swiss national air pollution monitoring network, NABEL. Sample preparation and the chemical analysis were performed on the AirOSol platform (IGE, Grenoble, France).
This research has been supported by the Agence Nationale de le Recherche (ANR ABS and Labex OSUG@2020, grant nos. ANR-21-CE01-0021-01 and ANR10-LABX56, respectively) and the Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (grant no. 202178).
This paper was edited by Bin Yuan and reviewed by two anonymous referees.
Aas, W., Salameh, T., Wegener, R., Hellén, H., Jaffrezo, J.-L., Roldin, P., Alonso-Blanco, E., Alastuey, A., Amelynck, C., Arduini, J., Bachmeier, F., Bergmans, B., Borbon, A., Bourtsoukidis, E., Butterfield, D., Buxbaum, I., Ceburnis, D., Claude, A., Colomb, A., Darfeuil, S., Dernie, J. Desservettaz, M., Díaz-Ramiro, E., Dufresne, M., Duval, M., Dury, M., Font, A., Fossum, K., Freney, E., Gangoiti, G., Ge, Y., Gomez, M. C., Gohy, M., Gros, V., Holubová, A., Jensen, N., Jokinen, T., Jones, M., Kesper, L., Klemp, D., Kubistin, D., Marinoni, A., Ngoc Thuy Dinh, V., Petäjä, T., Portillo-Estrada, M., Přívozníková, J., Putaud, J.-P., Rabe, R., Reimann, S., Riffault, V., Ritchie, S., Robins, C., Rodríguez de Torres, B. A., Poulain, L., Rüdiger, J., Sanock, A., Saez de Camara Oleaga, E., Schoon, N., Seco, R., Simmons, I., Simon, L., Simpson, D., Tison, E., Thomasson, A., Tsyro, S., Twigg, M., Tykkä, T., Verreyken, B., Yáñez-Serrano, A. M., Solberg, S., Yeung, K., Ylivinkka, I., Yttri, K. E., Vana, M., Watne, Å., and Williams, K.: Spatial Variability of VOCs, Ozone, and Carbonaceous Aerosols during the 2022 European Summer Heatwave, in preparation, 2025.
Amato, P., Brisebois, E., Draghi, M., Duchaine, C., Fröhlich-Nowoisky, J., Huffman, J. A., Mainelis, G., Robine, E., and Thibaudon, M.: Main Biological Aerosols, Specificities, Abundance, and Diversity, in: Microbiology of Aerosols, John Wiley & Sons, Ltd, 1–21, https://doi.org/10.1002/9781119132318.ch1a, 2017.
Baduel, C., Voisin, D., and Jaffrezo, J. L.: Comparison of analytical methods for Humic Like Substances (HULIS) measurements in atmospheric particles, Atmos. Chem. Phys., 9, 5949–5962, https://doi.org/10.5194/acp-9-5949-2009, 2009.
Bagnato, G., Iulianelli, A., Sanna, A., and Basile, A.: Glycerol Production and Transformation: A Critical Review with Particular Emphasis on Glycerol Reforming Reaction for Producing Hydrogen in Conventional and Membrane Reactors, Membranes, 7, 17, https://doi.org/10.3390/membranes7020017, 2017.
Bianco, A., Pailler, L., Joly, M., Delort, A.-M., Darfeuil, S., Jaffrezo, J.-L., and Deguillaume, L.: Sugars in clouds: Measurements and modelling investigation of their aqueous photodegradation, Atmos. Environ., 350, 121167, https://doi.org/10.1016/j.atmosenv.2025.121167, 2025.
Bonvalot, L., Tuna, T., Fagault, Y., Jaffrezo, J.-L., Jacob, V., Chevrier, F., and Bard, E.: Estimating contributions from biomass burning, fossil fuel combustion, and biogenic carbon to carbonaceous aerosols in the Valley of Chamonix: a dual approach based on radiocarbon and levoglucosan, Atmos. Chem. Phys., 16, 13753–13772, https://doi.org/10.5194/acp-16-13753-2016, 2016.
Borlaza, L. J. S., Weber, S., Jaffrezo, J.-L., Houdier, S., Slama, R., Rieux, C., Albinet, A., Micallef, S., Trébluchon, C., and Uzu, G.: Disparities in particulate matter (PM10) origins and oxidative potential at a city scale (Grenoble, France) – Part 2: Sources of PM10 oxidative potential using multiple linear regression analysis and the predictive applicability of multilayer perceptron neural network analysis, Atmos. Chem. Phys., 21, 9719–9739, https://doi.org/10.5194/acp-21-9719-2021, 2021.
Bukowiecki, N., Weingartner, E., Gysel, M., Coen, M. C., Zieger, P., Herrmann, E., Steinbacher, M., Gäggeler, H. W., and Baltensperger, U.: A Review of More than 20 Years of Aerosol Observation at the High Altitude Research Station Jungfraujoch, Switzerland (3580 m asl), Aerosol Air Qual. Res., 16, 764–788, https://doi.org/10.4209/aaqr.2015.05.0305, 2016.
Ciarelli, G., Aksoyoglu, S., Crippa, M., Jimenez, J.-L., Nemitz, E., Sellegri, K., Äijälä, M., Carbone, S., Mohr, C., O'Dowd, C., Poulain, L., Baltensperger, U., and Prévôt, A. S. H.: Evaluation of European air quality modelled by CAMx including the volatility basis set scheme, Atmos. Chem. Phys., 16, 10313–10332, https://doi.org/10.5194/acp-16-10313-2016, 2016.
Cruaud, P., Schivalocchi, F., Crouzet, A., Sanchez-Cid, C., Uzu, G., Tusha, D., Darfeuil, S., Jaffrezo, J.-L., Martins, J. M. F., and Larose, C.: Vegetation and Organic Matter Biodegradation Shape Atmospheric Sugar Concentrations and Airborne Microbial Communities, in preparation, 2025.
Dominutti, P. A., Borlaza, L. J. S., Sauvain, J.-J., Thuy, V. D. N., Houdier, S., Suarez, G., Jaffrezo, J.-L., Tobin, S., Trébuchon, C., Socquet, S., Moussu, E., Mary, G., and Uzu, G.: Source apportionment of oxidative potential depends on the choice of the assay: insights into 5 protocols comparison and implications for mitigation measures, Environ. Sci. Atmospheres, 3, 1497–1512, https://doi.org/10.1039/D3EA00007A, 2023.
EFSA Panel on Food Additives and Flavourings (FAF), Younes, M., Aquilina, G., Castle, L., Engel, K.-H., Fowler, P., Frutos Fernandez, M. J., Gundert-Remy, U., Gürtler, R., Husøy, T., Manco, M., Mennes, W., Moldeus, P., Passamonti, S., Shah, R., Waalkens-Berendsen, I., Wölfle, D., Wright, M., Cheyns, K., Mirat, M., Rincon, A. M., and Fürst, P.: Follow-up of the re-evaluation of glycerol (E 422) as a food additive, EFSA J., 20, e07353, https://doi.org/10.2903/j.efsa.2022.7353, 2022.
Elbert, W., Taylor, P. E., Andreae, M. O., and Pöschl, U.: Contribution of fungi to primary biogenic aerosols in the atmosphere: wet and dry discharged spores, carbohydrates, and inorganic ions, Atmos. Chem. Phys., 7, 4569–4588, https://doi.org/10.5194/acp-7-4569-2007, 2007.
Engling, G., Carrico, C. M., Kreidenweis, S. M., Collett, J. L., Day, D. E., Malm, W. C., Lincoln, E., Min Hao, W., Iinuma, Y., and Herrmann, H.: Determination of levoglucosan in biomass combustion aerosol by high-performance anion-exchange chromatography with pulsed amperometric detection, Atmos. Environ., 40, 299–311, https://doi.org/10.1016/j.atmosenv.2005.12.069, 2006.
Fröhlich, R., Cubison, M. J., Slowik, J. G., Bukowiecki, N., Canonaco, F., Croteau, P. L., Gysel, M., Henne, S., Herrmann, E., Jayne, J. T., Steinbacher, M., Worsnop, D. R., Baltensperger, U., and Prévôt, A. S. H.: Fourteen months of on-line measurements of the non-refractory submicron aerosol at the Jungfraujoch (3580 m a.s.l.) – chemical composition, origins and organic aerosol sources, Atmos. Chem. Phys., 15, 11373–11398, https://doi.org/10.5194/acp-15-11373-2015, 2015.
Georgelis, N., Fencil, K., and Richael, C. M.: Validation of a rapid and sensitive HPLC/MS method for measuring sucrose, fructose and glucose in plant tissues, Food Chem., 262, 191–198, https://doi.org/10.1016/j.foodchem.2018.04.051, 2018.
Gerber, D. W., Byerrum, R. U., Gee, R. W., and Tolbert, N. E.: Glycerol concentrations in crop plants, Plant Sci., 56, 31–38, https://doi.org/10.1016/0168-9452(88)90182-3, 1988.
Ghosh, S. K., Butler, M. S., and Lear, M. J.: Synthesis of 2-C-methylerythritols and 2-C-methylthreitols via enantiodivergent Sharpless dihydroxylation of trisubstituted olefins, Tetrahedron Lett., 53, 2706–2708, https://doi.org/10.1016/j.tetlet.2012.03.071, 2012.
Gika, H. G., Theodoridis, G. A., Vrhovsek, U., and Mattivi, F.: Quantitative profiling of polar primary metabolites using hydrophilic interaction ultrahigh performance liquid chromatography–tandem mass spectrometry, J. Chromatogr. A, 1259, 121–127, https://doi.org/10.1016/j.chroma.2012.02.010, 2012.
Glojek, K., Dinh Ngoc Thuy, V., Weber, S., Uzu, G., Manousakas, M., Elazzouzi, R., Džepina, K., Darfeuil, S., Ginot, P., Jaffrezo, J. L., Žabkar, R., Turšič, J., Podkoritnik, A., and Močnik, G.: Annual variation of source contributions to PM10 and oxidative potential in a mountainous area with traffic, biomass burning, cement-plant and biogenic influences, Environ. Int., 189, 108787, https://doi.org/10.1016/j.envint.2024.108787, 2024.
Graham, B., Mayol-Bracero, O. L., Guyon, P., Roberts, G. C., Decesari, S., Facchini, M. C., Artaxo, P., Maenhaut, W., Köll, P., and Andreae, M. O.: Water-soluble organic compounds in biomass burning aerosols over Amazonia 1. Characterization by NMR and GC-MS, J. Geophys. Res. Atmospheres, 107, LBA 14-1–LBA 14-16, https://doi.org/10.1029/2001JD000336, 2002.
Herich, H., Gianini, M. F. D., Piot, C., Močnik, G., Jaffrezo, J.-L., Besombes, J.-L., Prévôt, A. S. H., and Hueglin, C.: Overview of the impact of wood burning emissions on carbonaceous aerosols and PM in large parts of the Alpine region, Atmos. Environ., 89, 64–75, https://doi.org/10.1016/j.atmosenv.2014.02.008, 2014.
Hueglin, C., Gehrig, R., Baltensperger, U., Gysel, M., Monn, C., and Vonmont, H.: Chemical characterisation of PM2.5, PM10 and coarse particles at urban, near-city and rural sites in Switzerland, Atmos. Environ., 39, 637–651, https://doi.org/10.1016/j.atmosenv.2004.10.027, 2005.
Hummel, M., Hoose, C., Gallagher, M., Healy, D. A., Huffman, J. A., O'Connor, D., Pöschl, U., Pöhlker, C., Robinson, N. H., Schnaiter, M., Sodeau, J. R., Stengel, M., Toprak, E., and Vogel, H.: Regional-scale simulations of fungal spore aerosols using an emission parameterization adapted to local measurements of fluorescent biological aerosol particles, Atmos. Chem. Phys., 15, 6127–6146, https://doi.org/10.5194/acp-15-6127-2015, 2015.
Ito, J., Herter, T., Baidoo, E. E. K., Lao, J., Vega-Sánchez, M. E., Michelle Smith-Moritz, A., Adams, P. D., Keasling, J. D., Usadel, B., Petzold, C. J., and Heazlewood, J. L.: Analysis of plant nucleotide sugars by hydrophilic interaction liquid chromatography and tandem mass spectrometry, Anal. Biochem., 448, 14–22, https://doi.org/10.1016/j.ab.2013.11.026, 2014.
Kang, M., Ren, L., Ren, H., Zhao, Y., Kawamura, K., Zhang, H., Wei, L., Sun, Y., Wang, Z., and Fu, P.: Primary Biogenic and Anthropogenic Sources of Organic Aerosols in Beijing, China: Insights from Saccharides and n-Alkanes, Environmental Pollution, 243, 1579–1587, https://doi.org/10.1016/j.envpol.2018.09.118, 2018.
Kubica, P.: Determination of Glycerol, Propylene Glycol, and Nicotine as the Main Components in Refill Liquids for Electronic Cigarettes, Molecules, 28, 4425, https://doi.org/10.3390/molecules28114425, 2023.
Lazare, S., Bechar, D., Fernie, A. R., Brotman, Y., and Zaccai, M.: The proof is in the bulb: glycerol influences key stages of lily development, Plant J., 97, 321–340, https://doi.org/10.1111/tpj.14122, 2019.
López-Ruiz, R., Romero-González, R., and Garrido Frenich, A.: Ultrahigh-pressure liquid chromatography-mass spectrometry: An overview of the last decade, TrAC Trends Anal. Chem., 118, 170–181, https://doi.org/10.1016/j.trac.2019.05.044, 2019.
Marynowski, L., Łupikasza, E., Dąbrowska-Zapart, K., Małarzewski, Ł., Niedźwiedź, T., and Simoneit, B. R. T.: Seasonal and vertical variability of saccharides and other organic tracers of PM10 in relation to weather conditions in an urban environment of Upper Silesia, Poland, Atmos. Environ., 242, 117849, https://doi.org/10.1016/j.atmosenv.2020.117849, 2020.
Michoud, V., Hallemans, E., Chiappini, L., Leoz-Garziandia, E., Colomb, A., Dusanter, S., Fronval, I., Gheusi, F., Jaffrezo, J.-L., Léonardis, T., Locoge, N., Marchand, N., Sauvage, S., Sciare, J., and Doussin, J.-F.: Molecular characterization of gaseous and particulate oxygenated compounds at a remote site in Cape Corsica in the western Mediterranean Basin, Atmos. Chem. Phys., 21, 8067–8088, https://doi.org/10.5194/acp-21-8067-2021, 2021.
Nguyen, P. K., Owens, J. E., Lowe, L. E., and Mooney, E. H.: Analysis of sugars and amino acids in aphid honeydew by hydrophilic interaction liquid chromatography – Mass spectrometry, MethodsX, 7, 101050, https://doi.org/10.1016/j.mex.2020.101050, 2020.
Nielsen, N. J., Granby, K., Hedegaard, R. V., and Skibsted, L. H.: A liquid chromatography – tandem mass spectrometry method for simultaneous analysis of acrylamide and the precursors, asparagine and reducing sugars in bread, Anal. Chim. Acta, 557, 211–220, https://doi.org/10.1016/j.aca.2005.09.077, 2006.
Oduber, F., Calvo, A. I., Castro, A., Alves, C., Blanco-Alegre, C., Fernández-González, D., Barata, J., Calzolai, G., Nava, S., Lucarelli, F., Nunes, T., Rodríguez, A., Vega-Maray, A. M., Valencia-Barrera, R. M., and Fraile, R.: One-year study of airborne sugar compounds: Cross-interpretation with other chemical species and meteorological conditions, Atmospheric Res., 251, 105417, https://doi.org/10.1016/j.atmosres.2020.105417, 2021.
Piot, C., Ngoc Thuy Dinh, V., Ginot, P., Darfeuil, S., Elazzouzi, R., Uzu, G., and Jaffrezo, J.-L.: PMF Study of PM Sources in a Multi-Year Ice-Core Record at Mont Blanc (France), in preparation, 2025.
Pismennõi, D., Kiritsenko, V., Marhivka, J., Kütt, M.-L., and Vilu, R.: Development and Optimisation of HILIC-LC-MS Method for Determination of Carbohydrates in Fermentation Samples, Molecules, 26, 3669, https://doi.org/10.3390/molecules26123669, 2021.
Rego, A., Jesus, S., Motta, C., Delgado, I., Teixeira, R., Galhano dos Santos, R., and Castanheira, I.: Quantification by LC-MS/MS of individual sugars in fruit juice consumed in Portugal, J. Phys. Conf. Ser., 1065, 232004, https://doi.org/10.1088/1742-6596/1065/23/232004, 2018.
Rémy, S., Kipling, Z., Flemming, J., Boucher, O., Nabat, P., Michou, M., Bozzo, A., Ades, M., Huijnen, V., Benedetti, A., Engelen, R., Peuch, V.-H., and Morcrette, J.-J.: Description and evaluation of the tropospheric aerosol scheme in the European Centre for Medium-Range Weather Forecasts (ECMWF) Integrated Forecasting System (IFS-AER, cycle 45R1), Geosci. Model Dev., 12, 4627–4659, https://doi.org/10.5194/gmd-12-4627-2019, 2019.
Samake, A., Uzu, G., Martins, J. M. F., Calas, A., Vince, E., Parat, S., and Jaffrezo, J. L.: The unexpected role of bioaerosols in the Oxidative Potential of PM, Sci. Rep., 7, 10978, https://doi.org/10.1038/s41598-017-11178-0, 2017.
Samaké, A., Jaffrezo, J.-L., Favez, O., Weber, S., Jacob, V., Albinet, A., Riffault, V., Perdrix, E., Waked, A., Golly, B., Salameh, D., Chevrier, F., Oliveira, D. M., Bonnaire, N., Besombes, J.-L., Martins, J. M. F., Conil, S., Guillaud, G., Mesbah, B., Rocq, B., Robic, P.-Y., Hulin, A., Le Meur, S., Descheemaecker, M., Chretien, E., Marchand, N., and Uzu, G.: Polyols and glucose particulate species as tracers of primary biogenic organic aerosols at 28 French sites, Atmos. Chem. Phys., 19, 3357–3374, https://doi.org/10.5194/acp-19-3357-2019, 2019.
Samaké, A., Bonin, A., Jaffrezo, J.-L., Taberlet, P., Weber, S., Uzu, G., Jacob, V., Conil, S., and Martins, J. M. F.: High levels of primary biogenic organic aerosols are driven by only a few plant-associated microbial taxa, Atmos. Chem. Phys., 20, 5609–5628, https://doi.org/10.5194/acp-20-5609-2020, 2020.
Schivalocchi, F., Vogel, T. M., Keuschnig, C., Tusha, D., Darfeuil, S., Jaffrezo, J.-L., Piot, C., and Larose, C.: Temporal Shifts in Sub-Zero Microbial Metabolism and Community Structure Drive Carbohydrate Processing in Seasonal Snowpacks, in preparation, 2025.
Tsai, Y.-H., Tsai, C.-W., and Tipple, C. A.: A validated method for the analysis of sugars and sugar alcohols related to explosives via liquid chromatography mass spectrometry (LC-MS) with post-column addition, FORENSIC Chem., 28, 100404, https://doi.org/10.1016/j.forc.2022.100404, 2022.
Vida, M., Foret, G., Siour, G., Couvidat, F., Favez, O., Uzu, G., Cholakian, A., Conil, S., Beekmann, M., and Jaffrezo, J.-L.: Modelling of atmospheric concentrations of fungal spores: a 2-year simulation over France using CHIMERE, Atmos. Chem. Phys., 24, 10601–10615, https://doi.org/10.5194/acp-24-10601-2024, 2024.
Vincenti, B., Paris, E., Carnevale, M., Palma, A., Guerriero, E., Borello, D., Paolini, V., and Gallucci, F.: Saccharides as Particulate Matter Tracers of Biomass Burning: A Review, Int. J. Environ. Res. Public. Health, 19, 4387, https://doi.org/10.3390/ijerph19074387, 2022.
Wang, T., Huang, R.-J., Li, Y., Chen, Q., Chen, Y., Yang, L., Guo, J., Ni, H., Hoffmann, T., Wang, X., and Mai, B.: One-year characterization of organic aerosol markers in urban Beijing: Seasonal variation and spatiotemporal comparison, Sci. Total Environ., 743, 140689, https://doi.org/10.1016/j.scitotenv.2020.140689, 2020.
Weber, S., Salameh, D., Albinet, A., Alleman, L. Y., Waked, A., Besombes, J.-L., Jacob, V., Guillaud, G., Meshbah, B., Rocq, B., Hulin, A., Dominik-Sègue, M., Chrétien, E., Jaffrezo, J.-L., and Favez, O.: Comparison of PM10 Sources Profiles at 15 French Sites Using a Harmonized Constrained Positive Matrix Factorization Approach, Atmosphere, 10, 310, https://doi.org/10.3390/atmos10060310, 2019.
Yttri, K. E., Dye, C., and Kiss, G.: Ambient aerosol concentrations of sugars and sugar-alcohols at four different sites in Norway, Atmos. Chem. Phys., 7, 4267–4279, https://doi.org/10.5194/acp-7-4267-2007, 2007.
Zangrando, R., Barbaro, E., Kirchgeorg, T., Vecchiato, M., Scalabrin, E., Radaelli, M., Đorđević, D., Barbante, C., and Gambaro, A.: Five primary sources of organic aerosols in the urban atmosphere of Belgrade (Serbia), Sci. Total Environ., 571, 1441–1453, https://doi.org/10.1016/j.scitotenv.2016.06.188, 2016.