the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Organosulfates in atmospheric aerosol: synthesis and quantitative analysis of PM2.5 from Xi'an, northwestern China
Ru-Jin Huang
Junji Cao
Yang Chen
Lu Yang
Jincan Shen
Qihua You
Chunshui Lin
Bo Gao
Yongjie Li
Thorsten Hoffmann
Colin D. O'Dowd
Merete Bilde
Marianne Glasius
The sources, formation mechanism and amount of organosulfates (OS) in atmospheric aerosol are not yet well understood, partly due to the lack of authentic standards for quantification. In this study, we report an improved robust procedure for the synthesis of organosulfates with different functional groups. Nine authentic organosulfate standards were synthesized and four standards (benzyl sulfate, phenyl sulfate, glycolic acid sulfate, and hydroxyacetone sulfate) were used to quantify their ambient concentrations. The authentic standards and ambient aerosol samples were analyzed using an optimized ultra performance liquid chromatography–electrospray ionization-tandem mass spectrometric method (UPLC–ESI–MS/MS). The recovery ranged from 80.4 to 93.2 %, the limits of detection and limits of quantification obtained were 1.1–16.7 and 3.4–55.6 pg m−3, respectively. Measurements of ambient aerosol particle samples collected in winter 2013/2014 in urban Xi'an, northwestern China, show that glycolic acid sulfate (77.3 ± 49.2 ng m−3) is the most abundant species of the identified organosulfates followed by hydroxyacetone sulfate (1.3 ± 0.5 ng m−3), phenyl sulfate (0.14 ± 0.09 ng m−3), and benzyl sulfate (0.04 ± 0.01 ng m−3). Except for hydroxyacetone sulfate, which seems to form mainly from biogenic emissions in this region, the organosulfates quantified during winter in Xi'an show an increasing trend with an increase in the mass concentrations of organic carbon indicating their anthropogenic origin.
- Article
(1730 KB) - Full-text XML
-
Supplement
(320 KB) - BibTeX
- EndNote
Atmospheric aerosol particles represent a highly complex blend of inorganic and organic matter originating from a wide variety of both natural and anthropogenic sources. The organic fraction typically constitutes 20–90 % of the total submicron aerosol mass and is much less constrained in terms of chemical composition than the inorganic fraction (Jimenez et al., 2009; Hallquist et al., 2009). Only ∼ 10–30 % of the particulate organic matter has been identified as specific compounds despite years of effort and the use of the most sophisticated techniques available (Hoffmann et al., 2011). The insufficient knowledge of the composition of organic aerosol particles at the molecular level hinders a better understanding of the sources, formation and atmospheric processes of organic aerosol as well as their physicochemical properties and effects on climate and human health (Noziere et al., 2015).
Organosulfates are ubiquitous in atmospheric aerosol and have been detected in ambient aerosol particles from America, Europe, Asia and the Arctic during the last decade (e.g., Surratt et al., 2008; Iinuma et al., 2007; Stone et al., 2012; Hansen et al., 2014; Kourtchev et al., 2016; Surratt et al., 2007). Due to the presence of the deprotonated functional group R–O–, organosulfates are acidic and highly water soluble and thus can enhance the aerosol hygroscopicity. These characteristics, together with the light-absorbing property of organosulfates, lead to potential impacts on climate (Lin et al., 2014).
Organosulfates are tracers of secondary organic aerosol (SOA) formation and have been demonstrated to be produced from heterogeneous and multiphase reactions (e.g., Surratt et al., 2008; Iinuma et al., 2007; Chan et al., 2011; Zhang et al., 2012). Chamber studies have found that the oxidation of biogenic volatile organic compounds (BVOCs) including isoprene, monoterpenes and sesquiterpenes can form organosulfates on acidified sulfate particles (e.g., Surratt et al., 2008; Iinuma et al., 2007; Chan et al., 2011; Zhang et al., 2012). A very recent study revealed a previously unrecognized pathway for organosulfate formation through the heterogeneous reaction of SO2 with the unsaturated bond in oleic acid (Shang et al., 2016). A number of biogenic organosulfates have been observed in ambient aerosol, in particular, isoprene-derived organosulfates (e.g., Kristensen et al., 2011; He et al., 2014; Liao et al., 2015; Budisulistiorini et al., 2015). A recent study reported the formation of aromatic organosulfates by photochemical oxidation of polycyclic aromatic hydrocarbons (PAHs) in the presence of sulfate seed particles (Riva et al., 2016). Aromatic organosulfates have also recently been observed in urban aerosol from different locations in Asia. The presence of aromatic organosulfates was first suggested by Stone et al. (2012) based on analysis of aerosol samples collected at four sites in Asia. Kundu et al. (2013) quantified benzyl sulfate () and identified its homologous series with increasing number of methylene groups ( and ) in Lahore, Pakistan. Furthermore, Staudt et al. (2014) synthesized phenyl sulfate, benzyl sulfate, 3- and 4-methylphenyl sulfate and 2-, 3-, and 4-methylbenzyl sulfate and quantified them in aerosols collected in urban samples from Lahore and Pasadena, USA as well as Nepal. Ma et al. (2014) reported the contribution up to 64 % from aromatic organosulfates to the sum of identified organosulfates during winter in Shanghai, while Wang et al. (2016) found aromatic organosulfates to constitute less than 22 % of the detected number of organosulfates in Shanghai, Nanjing and Wuhan.
Organosulfates have been estimated to contribute 5–10 % of the organic mass in fine particles in the USA (Tolocka and Turpin, 2012). However, quantification of organosulfates is a challenging task due to the lack of authentic standards and incomplete understanding of the sources, precursors and formation processes of organosulfates. To date, many studies of organosulfates have remained at the qualitative level, although a limited number of studies have provided quantitative or semi-quantitative analysis of certain organosulfates (e.g., Kundu et al., 2013; Staudt et al., 2014; Ma et al., 2014; Olson et al., 2011; Hettiyadura et al., 2017). Moreover, several studies show that organosulfates are present as a wide range of species with individual species such as the organosulfate derived from isoprene epoxydiols (IEPOXs) contributing 0.2–1.4 % of the total organic aerosol mass (Liao et al., 2015). This further complicates the quantification of organosulfates. A few organosulfate standards have been synthesized for quantification purposes. For example, Olsen et al. (2011) measured 0.4–3.8 ng m−3 lactic acid sulfate and 1.9–11.3 ng m−3 glycolic acid sulfate in samples of PM2.5 (particulate matter with an aerodynamic diameter < 2.5 µm) from the US, Mexico City, and Pakistan. Kundu et al. (2013) measured monthly average concentrations of benzyl sulfate ranging from 0.05 to 0.5 ng m−3 in PM2.5 samples from Lahore, Pakistan. Staudt et al. (2014) quantified benzyl sulfate ranging from 4 to 90 pg m−3 in PM2.5 samples from Lahore (Pakistan), Godavari (Nepal), and Pasadena (California), while methylbenzyl sulfates, phenyl sulfate, and methylphenyl sulfates were observed intermittently in these three locations. Furthermore, Hettiyadura et al. (2015) developed a hydrophilic interaction liquid chromatography method using an amide stationary phase providing excellent retention of carboxy-organosulfates and isoprene-derived organosulfates, which was validated using six model organosulfates including aliphatic and aromatic organosulfates.
Previous field studies focusing on organosulfates were conducted mainly in Europe (e.g., Iinuma et al., 2007; Kristensen et al., 2011; Gómez-González et al., 2008, 2012; Nguyen et al., 2014; Martinsson et al., 2017), North America (e.g., Surratt et al., 2007; Nguyen et al., 2012; Worton et al., 2011) and a few in China (He et al., 2014; Ma et al., 2014). The particulate air pollution has been a serious environmental problem during recent winters in China, characterized by high secondary aerosol concentrations including sulfate and SOA (e.g., Huang et al., 2014; Elser et al., 2016; Wang et al., 2017). As organosulfates are tracers for SOA, more studies on organosulfates will help to better understand and constrain the SOA formation mechanisms in highly polluted regions (e.g., China) and to reconcile the underestimation of particle-phase organic carbon in atmospheric models.
In this study, nine organosulfate standards (phenyl sulfate, 3-methylphenyl sulfate, benzyl sulfate, 2-methyl benzyl sulfate, 3-methyl benzyl sulfate, 2, 4-dimethyl benzyl sulfate, 3, 5-dimethyl benzyl sulfate, hydroxyacetone sulfate and glycolic acid sulfate) were synthesized using an approach modified from Staudt et al. (2014) and Hettiyadura et al. (2015). These authentic standards were used to optimize an ultra performance liquid chromatography electrospray ionization-tandem mass spectrometric method (UPLC–ESI–MS/MS) for the quantification of organosulfates. The presence and concentration of four of these organosulfates, namely, benzyl sulfate, phenyl sulfate, glycolic acid sulfate and hydroxyacetone sulfate, were determined in ambient PM2,5 collected in urban air in Xi'an, China. The rest five organosulfates were not quantified in ambient PM2.5 because the standards were synthesized at a later stage of the study.
2.1 Chemicals and synthesis
The chemicals used for the synthesis of organosulfates included hydroxyacetone (99 %, Sigma Aldrich), glycolic acid (99 %, Sigma Aldrich), phenol (99.5 %, Tic), benzyl alcohol (99.8 %, Aladdin, Shanghai, China), m-cresol (99 %, Sigma Aldrich), sulfur trioxide pyridine complex (98 %, Sigma Aldrich), pyridine (99.9 %, Sigma Aldrich) and Dowex® 50WX8 (hydrogen form, 100–200 mesh, Sigma Aldrich). MilliQ water (18.2 MΩ) was used, and all other reagents were analytical grade and used without further purification.
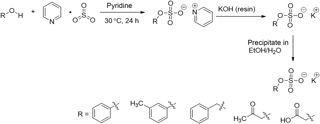
The organosulfate standards were synthesized using a general approach modified from Staudt et al. (2014) and Hettiyadura et al. (2015). Figure 1 shows the reaction scheme. In general, alcohol (7.0 mmol) and sulfur trioxide pyridine complex (1.2 equiv.) was dissolved in dry pyridine (10 mL) in an oven-dried, three-necked flask provided with magnetic stirring under nitrogen. The reaction mixture was stirred at 30 ∘C for 24 h, and then the solvent was removed via distillation under vacuum at 50 ∘C. The residue was redissolved in distilled water (10 mL) and titrated with 0.9 M KOH until pH was above 12. Neat ethanol (40 mL, 65 ∘C) was added to the aqueous solution. The resulting solution was heated to reflux followed by a quick vacuum filtration to remove the stark white precipitate. The mother liquor was then placed in a freezer (−25 ∘C) overnight. The potassium salts of organosulfate formed in the mother liquor were collected by vacuum filtration, rinsed with cold ethanol three times and dried to obtain the target product. The synthesized organosulfate standards were stored in refrigerator (∼ 4 ∘C) and no decomposition was observed after 2 years as confirmed by nuclear magnetic resonance (NMR) analysis.
2.2 Characterization
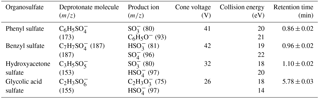
The synthesized products were characterized with NMR and ESI–MS. 1H NMR and 13C NMR spectra were recorded on a Bruker Advance-III 40 MHz spectrometer at 400 and 100 MHz, respectively using trimethylsilane (TMS) as an internal standard. Chemical shifts are reported in ppm downfield from the internal reference. The NMR spectra are shown in Supplement. The following abbreviations are used for the multiplicities: s = singlet, m = multiplet. The yield for phenyl sulfate was 45 %, 1H NMR (400 MHz, D2O): δ/ppm 7.29–7.43 (m, 5H), 13C NMR (100 MHz, D2O): δ/ppm 121.6, 126.4, 129.8 and 151.2. The yield for benzyl sulfate was 70 %, 1H NMR (400 MHz, DMSO-d6): δ/ppm 7.25–7.40 (m, 5 H), 4.76 (s, 2 H), 13C NMR (100 MHz, DMSO-d6): δ/ppm 67.9, 127.8, 128.0, 128.6 and 138.4. The yield for hydroxyacetone sulfate was 45 %, 1H NMR (400 MHz, DMSO-d6): δ/ppm 4.22 (s, 2 H), 2.11 (s, 3 H), 13C NMR (100 MHz, DMSO-d6): δ/ppm 26.9, 71.4 and 207.0. The yield for glycolic acid sulfate was 35 %, 1H NMR (400 MHz, DMSO-d6): δ/ppm 4.07 (s, 2 H) and 13C NMR (100 MHz, DMSO-d6): δ/ppm 65.0, 173.1. The organosulfate standards were recrystallized in ethanol for purification and purity of these synthesized standards is > 95 %, confirmed by NMR analysis. Exact mass spectra were recorded on a high-resolution mass spectrometer (HR–MS, Q Exactive Plus, Thermo Scientific, USA) equipped with an ESI source in the negative ion mode (ESI-). The ESI conditions were as follows: spray voltage −3.2 kV, collision energy (CE) 40 V for benzyl sulfate and 45 V for hydroxyacetone sulfate, 3-methylphenyl sulfate, glycolic acid sulfate and phenyl sulfate, capillary temperature 350 ∘C, aux gas heater temperature 320 ∘C, sheath gas flow rate 35 and aux gas flow rate 10. The mass resolving power was 70 000. Data acquisition was performed with m∕z ranging from 50 to 200.
2.3 PM2.5 samples
The 24 h integrated PM2.5 samples were collected on pre-baked (780 ∘C, 3 h) quartz-fiber filters (8 × 10 inch, Whatman, QM-A, USA) using a high-volume sampler (Tisch, Cleveland, OH, USA) at a flow rate of 1.05 m3 min−1 from 18 December 2013 to 17 February 2014. After collection, the filter samples were immediately wrapped in pre-baked aluminum foil and stored in a freezer (below −20 ∘C) until analysis. The sampling site was located on the rooftop of the Institute of Earth and Environment (∼ 10 m above the ground), Chinese Academy of Sciences (IEECAS, 34.23∘ N, 108.88∘ E), which is surrounded by residential, commercial and trafficked areas.
2.4 Sample analysis
A portion of the filter (6 × 0.526 cm2 punch) taken from each sample was sonicated for 25 min in 9 mL of acetonitrile (ACN)/water mixture (95:5, V∕V). The extracts were filtered through a 0.22 µm polypropylene membrane syringe filter to remove insoluble material. The eluate was concentrated almost to dryness with a gentle stream of purified nitrogen (99.999 %) at 45 ∘C using an evaporation system (TurboVap® LV, biotage), then redissolved in 500 µL of acetonitrile/water mixture (V∕V, 95:5). The prepared samples were stored at 4 ∘C in the refrigerator and analyzed within 24 h. The separation and quantification were realized using a ACQUITY UPLC system (equipped with a quaternary pump, autosampler, and thermostated column compartment) coupled to a tandem mass spectrometer (Xevo TQ MS, Waters, USA). The separation was carried out using a BEH amide column (2.1 mm × 100 mm, 1.7 µm particle size, Waters, USA) equipped with a pre-column. The column was maintained at 35 ∘C and the flow rate of mobile phase was 0.25 mL min−1. A 5 µL injection volume was used for quantitative analysis of samples and standards. The optimized mobile phase A (organic) consisted of ammonium acetate buffer (5 mM, pH 8.5) in ACN and ultra-pure water (95:5, V∕V) and mobile phase B (aqueous) consisted of ammonium acetate buffer (5 mM, pH 9) in ultra-pure water. A mobile phase gradient was used: mobile phase A was maintained at 98 % for 2 min, then decreased to 60 % from 2 to 5 min and then held there for 2 min; from 7 to 12 min mobile phase A was returned to 98 %. Organosulfates were detected by a TQ MS equipped with an ESI source in the negative ion mode. The mass spectrometer was operated in multiple reaction monitoring (MRM) mode. Optimized MS conditions for the four organosulfates chosen for the field studies (e.g., cone voltages and collision energies) are listed in Table 1. The capillary voltage was 2.7 kV, source temperature was 150 ∘C, desolvation temperature was 350 ∘C, desolvation gas (N2) flow rate at 800 L h−1, cone gas (N2) flow rate was 150 L h−1, and collision gas (Ar) flow rate was 0.16 mL min−1. All data were acquired and processed using MassLynx software (version 4.1). All samples and standard spectra were background subtracted.
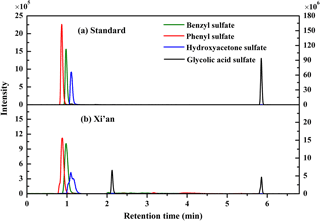
Figure 3Typical chromatograms of organosulfates from the mixture of authentic standard solution and ambient PM2.5 samples, measured with the UPLC–ESI–MS/MS method. Note, the intensity of benzyl sulfate and phenyl sulfate refers to the left y axis, and the intensity of hydroxyacetone sulfate and glycolic acid sulfate refers to right y axis.
Table 2Analytical performance of the UPLC-ESI-MS/MS method for organosulfate analysis.
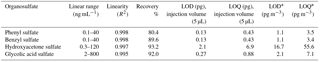
* For analyzing 6 × 0.526 cm punches of filters collected with high-volume samplers (sampling at 1.13 m3 min−1 for 24 h on 8” × 10” filters).
2.5 Quality control
For every 10 analyses, a procedural blank and a spiked sample, real ambient samples spiked with known amounts of a standard solution of organosulfates to be quantified, were measured to check for interference and cross-contamination. The external standard method was used for quantitative determination of the analytes. The limits of detection are defined as the minimum detectable peaks of individual species with a signal-to-noise (S/N) ratio of 3:1. The recoveries were determined by the analysis of the spiked samples: we first measured a filter punch without spike and then measured the second punch from the identical filter spiked with known amounts of a standard solution of organosulfates. The differences between these two measurements were divided by the amounts of organosulfates spiked to calculate the recoveries of individual organosulfates. This recovery test also provides an indication of potential matrix effect. The reproducibility (relative standard deviation, RSD) was determined by measuring five identical samples that were subjected to the same pretreatment procedure. The field blank samples were collected and analyzed, and the data reported here were corrected for the field blanks.
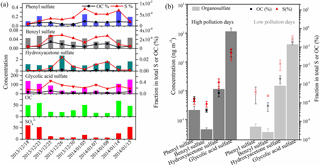
Figure 4Time series of organosulfates (ng m−3), OC (µg m−3), SO (µg m−3) and the fraction of individual organosulfates in total sulfur and OC (a). The average concentrations of individual organosulfates and the fractional contribution in total sulfur and OC during high and low pollution days are also shown (b).
3.1 Mass spectral fragmentation and UPLC separation
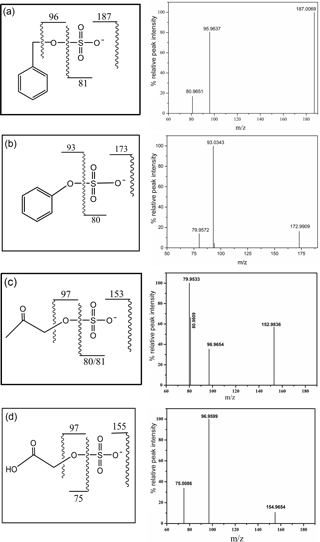
Each synthesized organosulfate was analyzed by high resolution tandem MS (MS/MS). The molecular ion for each organosulfate was assigned to the deprotonated molecule (R–O–). Major sulfur-containing product ions included the sulfite ion radical (• , m∕z 80) that is formed from the homolytic cleavage of the O–S bond, the sulfate ion radical (• , m∕z 96) that is formed from the homolytic cleavage of the C–O bond, the bisulfite anion (, m∕z 81) that is formed from hydrogen abstraction followed by the heterolytic cleavage of the O–S bond, and the bisulfate anion (, m∕z 97). Phenyl sulfate, 3-methylphenyl sulfate, and glycolic acid sulfate produce phenoxide (C6H5O−, m∕z 93), 3-methylphenoxide (C7H7O−, m∕z 107) and glycolate (, m∕z 75) anions, respectively, formed from neutral loss of SO3. The mass spectra of these compounds are shown in Fig. 2. The mass spectrum of phenyl sulfate is similar to that reported by Staudt et al. (2014), the mass spectra of hydroxy acetone sulfate and glycolic acid sulfate are similar to those reported by Hettiyadura et al. (2015) and the spectrum of benzyl sulfate is similar to that reported by Kundu et al. (2013), confirming the identity of the compounds.
The ESI-MS/MS in MRM mode is applied for the quantification of individual organosulfates. This can greatly enhance the selectivity and sensitivity by monitoring a transition pair of precursor and product ions and thus eliminating potential interferences from the complex aerosol matrix. Table 1 shows the optimized ESI- conditions and the transition pairs for each organosulfate studied. The organosulfate standards were separated by UPLC using a BEH amide column that retains extremely polar compounds through ionic, hydrogen bonding and dipole interactions. A gradient elution procedure was applied and the aqueous portion of the mobile phase increased from 7–43 %, leading to the baseline separation of four organosulfates within 6 min (Fig. 3a). The retention time was 0.86 min for phenyl sulfate, 0.96 min for benzyl sulfate, 1.10 min for hydroxyacetone sulfate and 5.78 min for glycolic acid sulfate, respectively. The mobile phase was buffered to slightly basic pH to maintain the deprotonated state of the organosulfates, which favors the separation. The amide functionalization of the BEH stationary phase introduces hydrogen bonding and strengthens interaction with organosulfates particularly for those containing carboxyl and hydroxyl functional groups. It should be noted that the chromatographic peak-broadening occurred particularly for phenyl sulfate and hydroxyacetone sulfate when analyzing the ambient samples (Fig. 3b). This might be explained by matrix effects due to the complex samples, which can influence the partitioning of analyte between the stationary phase and mobile phase, particularly for those analytes with weak retention on the column. However, the quantification of organosulfates is not affected by the peak broadening because the transition pair of precursor and product ions used in the MRM mode of the mass spectrometer guarantees selectivity and accuracy.
3.2 Method validation
Table 2 shows the analytical performance of the method under optimized UPLC and MS/MS conditions. The calibration curves of each organosulfate are highly linear (R2≥ 0.995), ranging from 0.1–40 ng mL−1 for phenyl sulfate and benzyl sulfate, from 0.3–120 ng mL−1 for hydroxyacetone sulfate, and 2.0–800 ng mL−1 for glycolic acid sulfate. The recoveries, determined by analyzing ambient samples spiked with known amounts of organosulfate standards, ranging from 80.4–93.2 %. The good recoveries indicate high extraction efficiency, low sample matrix effect and low error from sample pretreatment and the UPLC-MS measurement. The limit of detection (LOD, S/N = 3) and limit of quantification (LOQ, S/N = 10) ranged from 0.03 to 0.42 ng mL−1 and 0.09 to 1.4 ng mL−1 of the extracts, respectively. This corresponds to LODs of 1.1 to 16.7 pg m−3 and LOQs of 3.4 to 55.6 pg m−3, respectively, using the current set-up (see experimental section).
3.3 Quantification of organosulfates in ambient aerosol
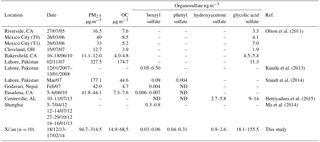
Ambient PM2.5 samples were extracted and analyzed by UPLC-MS/MS following the same procedure as the OS standards. The four selected organosulfates were identified according to the transition pairs of precursor and product ions of individual compounds on the MS/MS as well as the UPLC retention time. Table 3 shows the concentrations of phenyl sulfate, benzyl sulfate, hydroxyacetone sulfate, and glycolic acid sulfate in PM2.5 samples collected at Xi'an (this work), together with concentrations reported in the literature from other locations worldwide for comparison. In our samples from Xi'an glycolic acid sulfate (average 77.3 ± 49.2 ng m−3, range 18.1–155.5 ng m−3) was the most abundant species of the identified organosulfate followed by hydroxyacetone sulfate (average 1.3 ± 0.5 ng m−3, range 0.9–2.6 ng m−3), phenyl sulfate (average 0.14 ± 0.09 ng m−3, range 0.04–0.31 ng m−3) and benzyl sulfate (average 0.04 ± 0.01 ng m−3, range 0.03–0.06 ng m−3).
The concentration of glycolic acid sulfate quantified in this study is about one order of magnitude higher than those reported in the literature (see Table 3), indicating the substantial formation of this secondary organic compound in polluted urban Xi'an. Glycolic acid sulfate can form efficiently from glycolic acid relative to glyoxal in the presence of acidic sulfate particles (Olson et al., 2011). While both organic precursors (glycolic acid and glyoxal) have biogenic and anthropogenic origins, they form mainly from the oxidation of anthropogenic emissions during winter in Xi'an. The concentrations of particle-phase glyoxal and glycolic acid measured at Xi'an during winter have been reported to be significantly higher compared to other studied regions (e.g., Kawamura and Yasui, 2005; Miyazaki et al., 2009; Cheng et al., 2013), which thus may explain the elevated glycolic acid sulfate. The concentration of the other three organosulfates quantified in this study was much lower, but falling into the ranges measured in other regions.
It is noted that the time series of glycolic acid sulfate, phenyl sulfate and benzyl sulfate is similar to that of organic carbon (OC) and , while the concentration of hydroxyacetone sulfate did not show an increasing trend when the concentrations of OC increased (Fig. 4a). Hydroxyacetone sulfate can form from photochemical oxidation of isoprene and/or isoprene ozonolysis in the presence of acidic sulfate aerosols (Surratt et al., 2008; Riva et al., 2015), although hydroxyacetone was also suggested to originate from anthropogenic emissions (e.g., biomass burning and fossil fuel combustion) (Hansen et al., 2014). Also, the formation rate of biogenic hydroxyacetone sulfate and anthropogenic hydroxyacetone sulfate may different. This may explain the lack of correlation between hydroxyacetone sulfate and OC during winter in Xi'an. The average concentrations of glycolic acid sulfate, phenyl sulfate and benzyl sulfate were 1.3–3.2× higher during high pollution days (PM2.5 range of 293.7–314.5 µg m−3 with an average of 300.6 µg m−3) than during low pollution days (PM2.5 range of 94.7–121.2 µg m−3 with an average of 106.4 µg m−3), while the average concentrations of hydroxyacetone sulfate were rather similar between high pollution days and low pollution days (Fig. 4b). These four organosulfates together account for 0.25 % of total sulfur and 0.05 % of OC, respectively.
Nine authentic organosulfate standards, including phenyl sulfate, 3-methylphenyl sulfate, benzyl sulfate, 2-methyl benzyl sulfate, 3-methyl benzyl sulfate, 2, 4-dimethyl benzyl sulfate, 3, 5-dimethyl benzyl sulfate, hydroxyacetone sulfate and glycolic acid sulfate, were synthesized in this study using an improved robust procedure. The synthesized compounds of benzyl sulfate, phenyl sulfate, glycolic acid sulfate, and hydroxyacetone sulfate were used as standards for quantification of these molecules in ambient PM2.5 samples. The other five organosulfate standards were synthesized, but not used for quantification of ambient samples in this study. An improved UPLC-ESI-MS/MS method was developed and optimized for the quantification. The recovery ranges from 80.4–93.2 %, and the limits of detection and limits of quantification obtained are 1.1–16.7 and 3.4–55.6 pg m−3, respectively. Measurements of PM2.5 samples from Xi'an show that glycolic acid sulfate (77.3 ± 49.2 ng m−3) is the most abundant organosulfate followed by hydroxyacetone sulfate (1.3 ± 0.5 ng m−3), phenyl sulfate (0.14 ± 0.09 ng m−3) and benzyl sulfate (0.04 ± 0.01 ng m−3). Glycolic acid sulfate, phenyl sulfate and benzyl sulfate show an increasing trend with the increase of OC concentrations indicating their anthropogenic origin.
Raw data used in this study are archived at the Institute of Earth Environment, Chinese Academy of Sciences, and are available on request by contacting the corresponding author.
The supplement related to this article is available online at: https://doi.org/10.5194/amt-11-3447-2018-supplement.
The authors declare that they have no conflict of interest.
This work was supported by the National Natural Science Foundation of China
(NSFC) under grant No. 91644219, No. 41650110488, the Minjiang Scholar
Program and the Carlsberg Foundation.
Edited by: Mingjin Tang
Reviewed by: two anonymous referees
Budisulistiorini, S. H., Li, X., Bairai, S. T., Renfro, J., Liu, Y., Liu, Y. J., McKinney, K. A., Martin, S. T., McNeill, V. F., Pye, H. O. T., Nenes, A., Neff, M. E., Stone, E. A., Mueller, S., Knote, C., Shaw, S. L., Zhang, Z., Gold, A., and Surratt, J. D.: Examining the effects of anthropogenic emissions on isoprene-derived secondary organic aerosol formation during the 2013 Southern Oxidant and Aerosol Study (SOAS) at the Look Rock, Tennessee ground site, Atmos. Chem. Phys., 15, 8871–8888, https://doi.org/10.5194/acp-15-8871-2015, 2015.
Chan, M. N., Surratt, J. D., Chan, A. W. H., Schilling, K., Offenberg, J. H., Lewandowski, M., Edney, E. O., Kleindienst, T. E., Jaoui, M., Edgerton, E. S., Tanner, R. L., Shaw, S. L., Zheng, M., Knipping, E. M., and Seinfeld, J. H.: Influence of aerosol acidity on the chemical composition of secondary organic aerosol from β-caryophyllene, Atmos. Chem. Phys., 11, 1735–1751, https://doi.org/10.5194/acp-11-1735-2011, 2011.
Cheng, C. L., Wang, G. H., Zhou, B. H., Meng, J. J., Li, J. J., Cao, J. J., and Xiao, S.: Comparison of Dicarboxylic Acids and Related Compounds in Aerosol Samples Collected in Xi'An, China During Haze and Clean Periods, Atmos. Environ., 81, 443–449, https://doi.org/10.1016/j.atmosenv.2013.09.013, 2013.
Elser, M., Huang, R.-J., Wolf, R., Slowik, J. G., Wang, Q., Canonaco, F., Li, G., Bozzetti, C., Daellenbach, K. R., Huang, Y., Zhang, R., Li, Z., Cao, J., Baltensperger, U., El-Haddad, I., and Prévôt, A. S. H.: New insights into PM2.5 chemical composition and sources in two major cities in China during extreme haze events using aerosol mass spectrometry, Atmos. Chem. Phys., 16, 3207–3225, https://doi.org/10.5194/acp-16-3207-2016, 2016.
Gómez-González, Y., Surratt, J. D., Cuyckens, F., Szmigielski, R., Vermeylen, R., Jaoui, M., Lewandowski, M., Offenberg, J. H., Kleindienst, T. E., and Edney, E. O.: Characterization of Organosulfates From the Photooxidation of Isoprene and Unsaturated Fatty Acids in Ambient Aerosol Using Liquid Chromatography/(-)Electrospray Ionization Mass Spectrometry, J. Mass Spectrom., 43, 371–382, https://doi.org/10.1002/jms.1329, 2008.
Gómez-González, Y., Wang, W., Vermeylen, R., Chi, X., Neirynck, J., Janssens, I. A., Maenhaut, W., and Claeys, M.: Chemical characterisation of atmospheric aerosols during a 2007 summer field campaign at Brasschaat, Belgium: sources and source processes of biogenic secondary organic aerosol, Atmos. Chem. Phys., 12, 125–138, https://doi.org/10.5194/acp-12-125-2012, 2012.
Hallquist, M., Wenger, J. C., Baltensperger, U., Rudich, Y., Simpson, D., Claeys, M., Dommen, J., Donahue, N. M., George, C., Goldstein, A. H., Hamilton, J. F., Herrmann, H., Hoffmann, T., Iinuma, Y., Jang, M., Jenkin, M. E., Jimenez, J. L., Kiendler-Scharr, A., Maenhaut, W., McFiggans, G., Mentel, Th. F., Monod, A., Prévôt, A. S. H., Seinfeld, J. H., Surratt, J. D., Szmigielski, R., and Wildt, J.: The formation, properties and impact of secondary organic aerosol: current and emerging issues, Atmos. Chem. Phys., 9, 5155–5236, https://doi.org/10.5194/acp-9-5155-2009, 2009.
Hansen, A. M. K., Kristensen, K., Nguyen, Q. T., Zare, A., Cozzi, F., Nøjgaard, J. K., Skov, H., Brandt, J., Christensen, J. H., Ström, J., Tunved, P., Krejci, R., and Glasius, M.: Organosulfates and organic acids in Arctic aerosols: speciation, annual variation and concentration levels, Atmos. Chem. Phys., 14, 7807–7823, https://doi.org/10.5194/acp-14-7807-2014, 2014.
He, Q. F., Ding, X., Wang, X. M., Yu, J. Z., Fu, X. X., Liu, T. Y., Zhang, Z., Xue, J., Chen, D. H., and Zhong, L. J.: Organosulfates From Pinene and Isoprene Over the Pearl River Delta, South China: Seasonal Variation and Implicationin Formation Mechanisms, Environ. Sci. Technol., 48, 9236–9245, https://doi.org/10.1021/es501299v, 2014.
Hettiyadura, A. P. S., Stone, E. A., Kundu, S., Baker, Z., Geddes, E., Richards, K., and Humphry, T.: Determination of atmospheric organosulfates using HILIC chromatography with MS detection, Atmos. Meas. Tech., 8, 2347–2358, https://doi.org/10.5194/amt-8-2347-2015, 2015.
Hettiyadura, A. P. S., Jayarathne, T., Baumann, K., Goldstein, A. H., de Gouw, J. A., Koss, A., Keutsch, F. N., Skog, K., and Stone, E. A.: Qualitative and quantitative analysis of atmospheric organosulfates in Centreville, Alabama, Atmos. Chem. Phys., 17, 1343–1359, https://doi.org/10.5194/acp-17-1343-2017, 2017.
Hoffmann, T., Huang, R. J., and Kalberer, M.: Atmospheric analytical chemistry, Anal. Chem., 83, 4649–4664, 2011.
Huang, R. J., Zhang, Y. L., Bozzetti, C., Ho, K. F., Cao, J. J., Han, Y. M., Daellenbach, K. R., Slowik, J. G., Platt, S. M., and Canonaco, F.: High Secondary Aerosol Contribution to Particulate Pollution During Haze Events in China, Nature, 514, 218–222, https://doi.org/10.1038/nature13774, 2014.
Iinuma, Y., Müller, C., Berndt, T., Böge, O., Claeys, M., and Herrmann, H.: Evidence for the Existence of Organosulfates from β-Pinene Ozonolysis in Ambient Secondary Organic Aerosol, Environ. Sci. Technol., 41, 6678–6683, https://doi.org/10.1021/es070938t, 2007.
Jimenez, J. L., Canagaratna, M. R., Donahue, N. M., Prevot, A. S. H., Zhang, Q., Kroll, J. H., DeCarlo, P. F., Allan, J. D., Coe, H., and Ng, N. L.: Evolution of Organic Aerosols in the Atmosphere, Science, 326, 1525–1529, https://doi.org/10.1126/science.1180353, 2009.
Kawamura, K. and Yasui, O.: Diurnal Changes in the Distribution of Dicarboxylic Acids, Ketocarboxylic Acids and Dicarbonyls in the Urban Tokyo Atmosphere, Atmos. Environ., 39, 1945–1960, https://doi.org/10.1016/j.atmosenv.2004.12.014, 2005.
Kourtchev, I., Godoi, R. H. M., Connors, S., Levine, J. G., Archibald, A. T., Godoi, A. F. L., Paralovo, S. L., Barbosa, C. G. G., Souza, R. A. F., Manzi, A. O., Seco, R., Sjostedt, S., Park, J.-H., Guenther, A., Kim, S., Smith, J., Martin, S. T., and Kalberer, M.: Molecular composition of organic aerosols in central Amazonia: an ultra-high-resolution mass spectrometry study, Atmos. Chem. Phys., 16, 11899–11913, https://doi.org/10.5194/acp-16-11899-2016, 2016.
Kristensen, K. and Glasius, M.: Organosulfates and Oxidation Products From Biogenic Hydrocarbons in Fine Aerosols From A Forest in North West Europe During Spring, Atmos. Environ., 45, 4546–4556, https://doi.org/10.1016/j.atmosenv.2011.05.063, 2011.
Kundu, S., Quraishi, T. A., Yu, G., Suarez, C., Keutsch, F. N., and Stone, E. A.: Evidence and quantitation of aromatic organosulfates in ambient aerosols in Lahore, Pakistan, Atmos. Chem. Phys., 13, 4865–4875, https://doi.org/10.5194/acp-13-4865-2013, 2013.
Liao, J., Froyd, K. D., Murphy, D. M., Keutsch, F. N., Yu, G., Wennberg, P. O., St Clair, J. M., Crounse, J. D., Wisthaler, A., and Mikoviny, T.: Airborne Measurements of Organosulfates Over the Continental US, J. Geophys. Res.-Atmos., 120, 2990–3005, https://doi.org/10.1002/2014JD022378, 2015.
Lin, Y. H., Budisulistiorini, H., Chu, K., Siejack, R. A., Zhang, H. F., Riva, M., Zhang, Z. F., Gold, A., Kautzman, K. E., and Surratt, J. D.: Light-Absorbing Oligomer Formation in Secondary Organic Aerosol from Reactive Uptake of Isoprene Epoxydiols, Environ. Sci. Technol., 48, 12012–12021, https://doi.org/10.1021/es503142b, 2014.
Ma, Y., Xu, X. K., Song, W. H., Geng, F. H., and Wang, L.: Seasonal and Diurnal Variations of Particulate Organosulfates in Urban Shanghai, China, Atmos. Environ., 85, 152–160, https://doi.org/10.1016/j.atmosenv.2013.12.017, 2014.
Martinsson, J., Monteil, G., Sporre, M. K., Kaldal Hansen, A. M., Kristensson, A., Eriksson Stenström, K., Swietlicki, E., and Glasius, M.: Exploring sources of biogenic secondary organic aerosol compounds using chemical analysis and the FLEXPART model, Atmos. Chem. Phys., 17, 11025–11040, https://doi.org/10.5194/acp-17-11025-2017, 2017.
Miyazaki, Y., Aggarwal, S. G., Singh, K., Gupta, P. K., and Kawamura, K.: Dicarboxylic Acids and Water-Soluble Organic Carbon in Aerosols in New Delhi, India, in Winter: Characteristics and Formation Processes, J. Geophys. Res.-Atmos., 114, 1–12, https://doi.org/10.1029/2009JD011790, 2009.
Nguyen, T. B., Lee, P. B., Updyke, K. M., Bones, D. L., Laskin, J., Laskin, A., and Nizkorodov, S. A.: Formation of Nitrogen-And Sulfur-Containing Light-Absorbing Compounds Accelerated by Evaporation of Water from Secondary Organic Aerosols, J. Geophys. Res.-Atmos., 117, 1–14, https://doi.org/10.1029/2011JD016944, 2012.
Nguyen, Q. T., Kristensen, T. B., Hansen, A. M. K., Skov, H., Bossi, R., Massling, A., Sørensen, L. L., Bilde, M., Glasius, M., and Nøjgaard, J. K.: Characterization of Humic-Like Substances in Arctic Aerosols, J. Geophys. Res.-Atmos., 119, 5011–5027, https://doi.org/10.1002/2013JD020144, 2014.
Noziere, B., Kalberer, M., Claeys, M., Allan, J., D'Anna, B., Decesari, S., Finessi, E., Glasius, M., Grgic, I., and Hamilton, J. F.: The Molecular Identification of Organic Compounds in the Atmosphere: State of the Art and Challenges, Chem. Rev., 115, 3919–3983, https://doi.org/10.1021/cr5003485, 2015.
Olson, C. N., Galloway, M. M., Yu, G., Hedman, C. J., Lockett, M. R., Yoon, T., Stone, E. A., Smith, L. M., and Keutsch, F. N.: Hydroxycarboxylic Acid-Derived Organosulfates: Synthesis, Stability, and Quantification in Ambient Aerosol, Environ. Sci. Technol., 45, 6468–6474, https://doi.org/10.1021/es201039p, 2011.
Riva, M., Tomaz, S., Cui, T. Q., Lin, Y. H., Perraudin, E., Gold, A., Stone, E. A., Villenave, E., and Surratt, J. D.: Evidence for an Unrecognized Secondary Anthropogenic Source of Organosulfates and Sulfonates: Gas-Phase Oxidation of Polycyclic Aromatic Hydrocarbons in the Presence of Sulfate Aerosol, Environ. Sci. Technol., 49, 6654–6664, https://doi.org/10.1021/acs.est.5b00836, 2015.
Riva, M., Budisulistiorini, S. H., Zhang, Z. F., Gold, A., and Surratt, J. D.: Chemical Characterization of Secondary Organic Aerosol Constituents From Isoprene Ozonolysis in the Presence of Acidic Aerosol, Atmos. Environ., 130, 5–13, https://doi.org/10.1016/j.atmosenv.2015.06.027, 2016.
Shang, J., Passananti, M., Dupart, Y., Ciuraru, R., Tinel, L., Rossignol, S. P., Perrier, S. B., Zhu, T., and George, C.: SO2 Uptake on Oleic Acid: A New Formation Pathway of Organosulfur Compounds in the Atmosphere, Environ. Sci. Tech. Let., 3, 67–72, https://doi.org/10.1021/acs.estlett.6b00006, 2016.
Staudt, S., Kundu, S., Lehmler, H. J., He, X. R., Cui, T. Q., Lin, Y. H., Kristensen, K., Glasius, M., Zhang, X. L., Weber, R. J., Surratt, J. D., and Stone, E. A.: Aromatic Organosulfates in Atmospheric Aerosols: Synthesis, Characterization, and Abundance, Atmos. Environ., 94, 366–373, https://doi.org/10.1016/j.atmosenv.2014.05.049, 2014.
Stone, E. A., Yang, L. M., Liya, E. Y., and Rupakheti, M.: Characterization of Organosulfates in Atmospheric Aerosols at Four Asian Locations, Atmos. Environ., 47, 323–329, https://doi.org/10.1016/j.atmosenv.2011.10.058, 2012.
Surratt, J. D., Kroll, J. H., Kleindienst, T. E., Edney, E. O., Claeys, M., Sorooshian, A., Ng, N. L., Offenberg, J. H., Lewandowski, M., and Jaoui, M.: Evidence for Organosulfates in Secondary Organic Aerosol, Environ. Sci. Technol., 41, 517–527, https://doi.org/10.1021/es062081q, 2007.
Surratt, J. D., Gómez-González, Y., Chan, A. W. H., Vermeylen, R., Shahgholi, M., Kleindienst, T. E., Edney, E. O., Offenberg, J. H., Lewandowski, M., and Jaoui, M.: Organosulfate Formation in Biogenic Secondary Organic Aerosol, J. Phys. Chem. A., 112, 8345–8378, https://doi.org/10.1021/jp802310p, 2008.
Tolocka, M. P. and Turpin, B.: Contribution of Organosulfur Compounds to Organic Aerosol Mass, Environ. Sci. Technol., 46, 7978–7983, https://doi.org/10.1021/es300651v, 2012.
Wang, X. K., Rossignol, S., Ma, Y., Yao, L., Wang, M. Y., Chen, J. M., George, C., and Wang, L.: Molecular Characterization of Atmospheric Particulate Organosulfates in Three Megacities at the Middle and Lower Reaches of the Yangtze River, Atmos. Chem. Phys., 16, 2285–2298, https://doi.org/10.5194/acp-16-2285-2016, 2016.
Wang, Y. C., Huang, R. J., Ni, H. Y., Chen, Y., Wang, Q. Y., Li, G. H., Tie, X. X., Shen, Z. X., Huang, Y., and Liu, S. X.: Chemical Composition, Sources and Secondary Processes of Aerosols in Baoji City of Northwest China, Atmos. Environ., 158, 128–137, https://doi.org/10.1016/j.atmosenv.2017.03.026, 2017.
Worton, D. R., Goldstein, A. H., Farmer, D. K., Docherty, K. S., Jimenez, J. L., Gilman, J. B., Kuster, W. C., de Gouw, J., Williams, B. J., Kreisberg, N. M., Hering, S. V., Bench, G., McKay, M., Kristensen, K., Glasius, M., Surratt, J. D., and Seinfeld, J. H.: Origins and composition of fine atmospheric carbonaceous aerosol in the Sierra Nevada Mountains, California, Atmos. Chem. Phys., 11, 10219–10241, https://doi.org/10.5194/acp-11-10219-2011, 2011.
Zhang, H. F., Worton, D. R., Lewandowski, M., Ortega, J., Rubitschun, C. L., Park, J. H., Kristensen, K., Campuzano-Jost, P., Day, D. A., and Jimenez, J. L.: Organosulfates as Tracers for Secondary Organic Aerosol (SOA) Formation From 2-Methyl-3-Buten-2-Ol (Mbo) in the Atmosphere, Environ. Sci. Technol., 46, 9437–9446, https://doi.org/10.1021/es301648z, 2012.