the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
A method for extracting calibrated volatility information from the FIGAERO-HR-ToF-CIMS and its experimental application
Thomas J. Bannan
Michael Le Breton
Michael Priestley
Stephen D. Worrall
Asan Bacak
Nicholas A. Marsden
Archit Mehra
Julia Hammes
Mattias Hallquist
M. Rami Alfarra
Ulrich K. Krieger
Jonathan P. Reid
John Jayne
Wade Robinson
Gordon McFiggans
Carl J. Percival
Dave Topping
The Filter Inlet for Gases and AEROsols (FIGAERO) is an inlet specifically designed to be coupled with the Aerodyne High-Resolution Time-of-Flight Chemical Ionization Mass Spectrometer (HR-ToF-CIMS). The FIGAERO-HR-ToF-CIMS provides simultaneous molecular information relating to both the gas- and particle-phase samples and has been used to extract vapour pressures (VPs) of the compounds desorbing from the filter whilst giving quantitative concentrations in the particle phase. However, such extraction of vapour pressures of the measured particle-phase components requires use of appropriate, well-defined, reference compounds. Vapour pressures for the homologous series of polyethylene glycols (PEG) (() for n=3 to n=8), covering a range of vapour pressures (VP) (10−1 to 10−7 Pa) that are atmospherically relevant, have been shown to be reproduced well by a range of different techniques, including Knudsen Effusion Mass Spectrometry (KEMS). This is the first homologous series of compounds for which a number of vapour pressure measurement techniques have been found to be in agreement, indicating the utility as a calibration standard, providing an ideal set of benchmark compounds for accurate characterization of the FIGAERO for extracting vapour pressure of measured compounds in chambers and the real atmosphere. To demonstrate this, single-component and mixture vapour pressure measurements are made using two FIGAERO-HR-ToF-CIMS instruments based on a new calibration determined from the PEG series. VP values extracted from both instruments agree well with those measured by KEMS and reported values from literature, validating this approach for extracting VP data from the FIGAERO. This method is then applied to chamber measurements, and the vapour pressures of known products are estimated.
- Article
(2437 KB) - Full-text XML
-
Supplement
(239 KB) - BibTeX
- EndNote
Trace gases and aerosol particles, from anthropogenic and natural sources, are important components of the Earth's climate system, the components of which vary significantly in terms of properties such as volatility, affecting their impact on air quality and climate change (Glasius and Goldstein, 2016). There are currently substantial uncertainties in many physicochemical parameters determining the loading, size, composition and properties of ambient atmospheric aerosol particles, including component vapour pressures (VPs; Bilde et al., 2015), that are required to predict their environmental and human health impacts. This is attributable in large part to the fact that a significant fraction of fine atmospheric aerosol particles are comprised of organic material (20 %–90 % of particle mass) (Jimenez et al., 2009), containing potentially thousands of mostly unidentified compounds with properties that are often not well known.
This organic aerosol is a major component of the fine particle mass in the atmosphere and is made up of primary organic aerosol (POA), which is emitted directly from sources such as industry, biomass burning and vehicle emissions but also secondary organic aerosol formed from the oxidation of gas-phase precursors (Robinson et al., 2007). Volatile organic compounds (VOCs), emitted from both natural and anthropogenic sources, are oxidized through two possible pathways, fragmentation and functionalization (Donahue et al., 2011). Functionalization can create compounds with a huge range of expected saturation vapour pressures between 0.1 and 10−10 Pa and lower (Jimenez et al., 2009; O'Meara et al., 2014; Bilde et al., 2015; Tröstl et al., 2016). The identity, concentrations and properties of such oxidation products are important in order to understand the formation of secondary organic aerosol (SOA) but also the general production of oxygenated compounds partitioning into existing SOA particles that can affect air quality in both outdoor and indoor environments. Uncertainties in the physicochemical properties of pure components and condensed-phase mixtures, as well as absolute composition, affect our ability to accurately predict this partitioning between the gas and particle phase and the subsequent effects on climate, air quality and fundamentally human health.
The equilibrium vapour pressure of each aerosol constituent is determined, in large part, by its pure component saturation VP. Saturation VPs of many organic components are poorly known, particularly for the least volatile compounds of interest for atmospheric purposes (Bilde et al., 2015). The importance of this fundamental property is discussed extensively in Bilde et al. (2015), and the sensitivity of predicted mass, composition and particle properties to uncertainties in VP vary according to the complexity of the system being studied, both with regards to the number of compounds used in partitioning and additional processes included in any model (Valorso et al., 2011; O'Meara et al., 2014; McVay et al., 2016). Single-component measurements of vapour pressures using instruments such as the Knudsen Effusion Mass Spectrometry (KEMS), following the methodology of Booth et al. (2009), have been recently reported (Booth et al., 2012; Bannan et al., 2017) and have been extended to consider vapour pressures in simple multicomponent systems (Booth et al., 2017). Such measurements are ongoing with the KEMS, focusing on atmospherically relevant compounds. Considerable uncertainty remains when extracting vapour pressure measurements from a single technique, with more work required to resolve the apparent discrepancies between techniques (Bilde et al., 2015). Studies reporting measurements of vapour pressure would benefit from an, at the time, unidentified series of reference standards, with volatility ranging across those accessible to the measurement techniques being deployed (Bilde et al., 2015). Following the recommendations of the Bilde et al. (2015) study and work within Topping et al. (2018), Krieger et al. (2018) identified the homologous series of polyethylene glycols (PEG; () for n=3 to n=8) as a series of compounds with vapour pressures exhibiting very good agreement (data was consistent with the 95 % confidence interval of a linear regression to all measurements) over a wide atmospherically relevant VP range when measured using different experimental methods. This series therefore provides an ideal benchmark for characterizing individual VP measurement techniques.
The High-Resolution Time-of-Flight Chemical Ionization Mass Spectrometer (HR-ToF-CIMS) coupled with the Filter Inlet for Gases and Aerosols (FIGAERO), hereafter referred to as the FIGAERO-CIMS, has the potential to provide compound-specific volatility information from ambient aerosol particles (Lopez-Hilfiker et al., 2014). The FIGAERO system was first introduced by Lopez-Hilfiker et al. (2014) and was subsequently commercialized by Aerodyne Research, Inc. (ARI) to be adaptable to the ToF-CIMS system. The FIGAERO inlet provides molecular determination of gas- and particle-phase samples. During the gas-phase measurement mode, particles from the aerosol sample are collected on a PTFE filter. After a period of collection, the filter is moved to the inlet of the instrument, and dry, heated nitrogen is passed through it to vaporize the particulate for analysis by the ToF-CIMS. The evolution of the MS signals from different compounds changes independently as a function of temperature, creating a thermogram that is m∕z-specific. The temperature for which the desorbed signal shows a maximum for each compound has been used previously to extract vapour pressure information in laboratory characterization (Lopez-Hilfiker et al., 2014) and field work studies (Lopez-Hilfiker et al., 2016; D'Ambro et al., 2017). A model framework has recently been developed to retrieve volatility and mass transport information from this inlet (Schobesberger et al., 2018). Such online analysis with high temporal resolution has the potential to improve our quantitative and detailed understanding of the diurnal evolution of gas- and particle-phase composition, and based on the use of this inlet to provide VP information, applying the series identified by the Krieger et al. (2018) study for calibrations will be of benefit to the accuracy of future derived measurements of this type.
In this study we will therefore demonstrate the use of this PEG series calibration dataset as a method for extracting quantitative vapour pressures from the FIGAERO inlet. The FIGAERO system used here is the version produced by ARI. Single-component measurements made with two separate ARI FIGAERO inlets for compounds of known VP are reported. The application of the FIGAERO to characterize the volatility of species produced in a chamber experiment is then described as a demonstration of the application of this method to a more complex matrix of components.
2.1 Choice of reference compounds
The vapour pressure of the polyethylene glycol (PEG) series, as described in the Krieger et al. (2018) study, were measured using multiple techniques: KEMS (Booth et al., 2009), electrodynamic balance instruments (Zardini et al., 2006; Rovelli et al., 2016) and the Tandem Differential Mobility Analyser (TDMA) including a laminar flow tube (Bilde et al., 2003). The reported vapour pressure of the PEG series demonstrated good agreement between these techniques over a wide range of VPs (spanning 5 orders of magnitude from about 10−1 to 10−7 Pa at room temperature). These measurements also compared well to data extrapolated from high temperatures, suggesting that the thermal energy utilized in techniques such as the FIGAERO will not lead to chemical modification of the target molecules. The physical state of the reference compound is important to consider when making VP measurements (Soonsin et al., 2010; Bilde et al., 2015). If the saturation vapour pressure of a compound is measured in the solid state, it needs to be converted to that of the subcooled liquid for use and interpretation within atmospheric models, which can add additional uncertainty through the required conversion. The PEG series therefore act as ideal reference materials as their members are all liquid at the temperatures at which the measurements are routinely performed.
The PEG compounds used in this study show no evidence of degradation with either the age or temperature at which the sample is measured. Measurement of PEG-4 VP by KEMS multiple times over a 6-month period showed no variation beyond measurement uncertainties, and data up to temperatures of 450 K reported in the literature are consistent with those measured at room temperature, demonstrating their thermal stability (Krieger et al., 2018). The stability of the PEG compounds allowed samples to be shared between the co-authors of Krieger et al. (2018), ensuring sample conformity.
As saturation vapour pressures of dicarboxylic acids have been determined with a large number of techniques and different instruments over a substantial temperature range, Bilde et al. (2015) evaluated the combined datasets, providing best estimates with uncertainty ranges for each of the straight-chain dicarboxylic acids. Therefore, these dicarboxylic acids are also used to validate the use of the PEG series as a calibration standard. A limitation of this approach is that the lowest Bilde et al. (2015) reference is pinic acid, and therefore testing of the full vapour pressure range provided by the PEG samples is limited to Pa with this approach. A small subsection of other literature values measured by one technique alone is used to extend this range in this study. It should be noted that measurements with the KEMS have suggested that samples of the dicarboxylic acids degrade over long periods of storage (>6 months) and can influence the measured vapour pressure. Appropriate storage and quick use of the chemicals, or appropriate purification methods, are therefore deemed essential for such measurement studies.
Tetraethylene glycol (PEG-4) (99 %) was purchased from Sigma-Aldrich, and PEG-5 to 8 were purchased from Polypure AS, Oslo, Norway, with purities of 99 % or higher and used with no further preparation. All PEG samples were stored in a fridge. Dicarboxylic acids were purchased from Sigma-Aldrich, again with purities of 99 % or higher and used with no further preparation. All dicarboxylic acids and samples used were measured within 1 month of receiving the samples and stored in accordance with the suppliers' recommendations.
2.2 FIGAERO-CIMS
This study utilized two FIGAERO-CIMS instruments, operated by the University of Manchester (UMan) and Gothenburg University (GU) groups. Both FIGAERO systems were manufactured by ARI and employ the ARI and Tofwerk High-resolution Time-of-Flight Chemical Ionization Mass Spectrometer (ToF-CIMS) instruments, similar to that described by Lee et al. (2014). The FIGAERO inlet was coupled to a reduced pressure ion molecule reaction (IMR) region, which is in turn coupled to a high-resolution time-of-flight mass spectrometer (APi-ToF) (Junninen et al., 2010). The ARI FIGAERO inlet used in this work is similar to that described by Lopez-Hilfiker et al. (2014). A brief description of the ARI FIGAERO system follows. The UMAN CIMS was operated with iodide as the regent ion, and the GU CIMS was operated with acetate or iodide as the reagent ion.
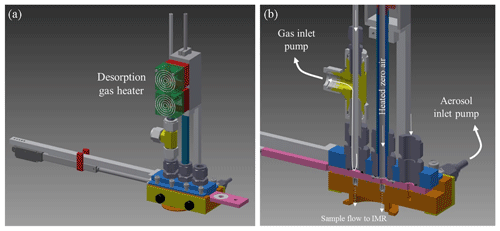
Figure 1Drawing of the ARI FIGAERO assembly. Panel (a) shows the full assembly with a mechanical actuator that controls gas sampling and aerosol collection or aerosol desorption operating modes. Panel (b) is a cross-sectional view that show flows for both gas and particle sampling mode and the two apertures that connect with the IMR. In this view the FIGAERO slide is positioned in the aerosol desorption mode, and the gas sample flow into the IMR is closed.
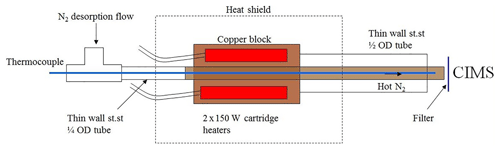
Figure 2Internal schematic of the FIGAERO desorption gas heater unit (“st.st” is for stainless steel). Temperature above the filter is measured at the end point of the heated tube by the long thermocouple running through the inlet, shown here in blue.
The ARI FIGAERO assembly is shown in Fig. 1. The FIGAERO is a two-port inlet, one dedicated to gas sampling (all Teflon) and the second dedicated to aerosol sampling (all metal). The FIGAERO couples both inlets with the chemical ionization region of the ToF MS. The FIGAERO operates in two modes, one being ambient air sampling for trace gas analysis with the CIMS while simultaneously collecting particles on a PFTE filter from a separate inlet. The second mode is the thermal desorption of the collected particles in nitrogen, allowing the detection of the desorbed vapours with the CIMS. When in the thermal desorption mode, the exclusively gas-phase port to the CIMS is blocked by the moveable tray, and the PTFE filter is moved to the exclusive port for thermal desorption. In this position 2 SLM of temperature-controlled nitrogen flow is delivered across the filter, desorbing the collected components from the filter. This is known as the “temperature ramp” phase. The evolution of the MS signals from different compounds during the temperature ramp phase changes independently as a function of temperature-creating thermograms that are m∕z-specific. Tmax is measured as the temperature just above the filter, as shown in Fig. 2. Two 150 W cartridge heaters are used to heat a copper block that connects with a 1∕4 in. OD copper tube. The nitrogen desorption gas is heated as it flows through this hot copper section, which is also nickel-plated. The combined length of the copper block and the 1∕4 in. copper tube is 16 cm and is set based on thermal modelling to provide maximum heat transfer for the ∼2 SLM N2 desorption gas flow maintained by a programmable mass flow controller. The gas temperature is measured by a 1∕16 in. diameter thermocouple positioned inside and just near the exit of the 1∕4 in. OD copper tube (∼5 mm above the PTFE Teflon filter, as detailed in Fig. 2). A 1∕2 in. OD stainless steel tube 14.6 cm in length is soldered to the copper heater block and provides thermal isolation and mechanical mounting of the heater unit to the FIGAERO assembly.
The temperature at which the desorbed signal for a compound reaches a maximum is used here to extract vapour pressure information. Once the temperature ramp phase is complete, under normal operating conditions, the nitrogen is then held at the maximum desorption temperature for a programmed period of time to ensure that all of the collected components have been removed from the filter, known as the “temperature soak” phase. After each ramp and soak phase, the heating is turned off and the unheated nitrogen is then used to cool the filter (cooling phase), allowing the filter to return to the starting temperature before the moveable tray switches back to trace gas analysis and before filter collection of particulate matter.
The UMan ToF-CIMS has been described in detail by Priestley et al. (2018a, b). The UMan FIGAERO-CIMS was exclusively run with iodide as the reagent ion during this study, as described in Reyes Villegas et al. (2018), and it was this system that measured the PEG series only. The GU CIMS (Faxon et al., 2018; Le Breton et al., 2018a, b) hardware is identical to that of the UMan CIMS, although tuning of the ion optics and flows differs to optimize the signal-to-noise ratio and total ion counts. Results from both acetate and iodide reagent ions from the GU FIGAERO-CIMS are presented here.
Prior to each sample measurement being made using both the GU and UMan FIGAERO-CIMS instruments, background measurements were obtained. First, a new filter was placed in the FIGAERO, and the temperature was ramped to 200 ∘C for 10 min to ensure the filter was clean and then cooled. A ramp, soak and cool cycle matching that of the subsequent sample was then completed to obtain the background. During the PEG series measurements, the filter was ramped to 200 ∘C (temperature above the filter) over a period of 20 min (at a rate of 8.75 ∘C min−1), held at 200 ∘C for 10 min and finally allowed to cool back to room temperature for a period of 5 min. The same cycle was used for the single-component measurements for both the GU and UMan instruments. It is however noted that the analysis provided here does not take into account the possibility of a change in ramp rate affecting the Tmax. It is therefore recommended that the calibration cycles match that of the measurements. Temperature cycles and gas flows were controlled using the ARI EyeOn™ control system.
Using the UMan FIGAERO, measurements of the PEG series were performed by first using a blank filter as a background then depositing the PEG sample on the Zefluor® PTFE membrane filter (2 µm pore size) for each desorption, cleaning it and then rerunning it with the next PEG sample. Four desorptions of each PEG were performed by depositing 0.1 µL of two different concentrations (two repeats of each), of 200 and 2000 µg cm−3, with a mean of the four desorptions being reported as the Tmax. No linear dependence of Tmax with concentration was observed across this concentration range. As with any calibration, it is recommended to use a comparable amount of calibrant material as would be expected to accumulate during the measurements, as it is noted that the amount of condensed material on the filter can affect the Tmax. A range of calibration concentrations larger than that reported in this study is suggested for future studies, and the small range is noted here as a limitation of this study. PEG calibrations were generally conducted individually and were manually syringed on to the filter. The reported Tmax values for one of the highest concentration runs for PEG 4 and PEG 6 as well as PEG 5 and PEG 8 were mixed in two separate experiments. The conditions were designed to ensure that the Tmax of the PEG series was not mixture-dependent, although a more detailed study is required. For the single-component measurements other than the PEG series, a known mass of the species to be calibrated is added to a solvent (methanol or deionized water) to create a known concentration in the solvent, and then 0.1 µL of it is placed onto the Zefluor® PTFE membrane filter using a syringe injector.
The FIGAERO-CIMS instrument analysis software (ARI Tofware version 2.5.11) was utilized to attain high-resolution 1 Hz time series of the compounds presented here. For the UMan CIMS, mass-to-charge calibration was performed for five known masses, I−, , , and , covering a mass range of 127 to 381 m∕z. The mass-to-charge calibration was fitted to a third-order polynomial and was accurate to within 2 ppm, ensuring peak identification was accurate below 3 ppm. The PEGs were detected as adducts in the UMan experiments, i.e. ), where n=4 to 8.
Due to the relativity small numbers of thermograms analysed from the UMan FIGAERO-CIMS, the Tmax values from the Manchester data were manually extracted. The average (mean) of the maximum three values in the thermogram is used to extract the Tmax values reported here. For the GU FIGAERO, thermograms were evaluated with the GUFIT (Gothenburg University FItting for Thermograms) procedure, which is described in detail in the Supplement. In this study only the Tmax is reported; however an alternative method, “T50”, as described in Stark et al. (2017), which uses the temperature at which 50 % of the signal is desorbed, could also have been employed here.
2.3 Chamber experiments
In addition to the PEG VP calibrations, we also performed FIGAERO measurements of secondary organic aerosols generated in the Manchester Photochemical Aerosol Chamber, and vapour pressures of several organic acids (mass accuracy all < 2 ppm) from measurements made in these experiments are reported here. Briefly, the chamber consists of an 18 m3 Teflon bag illuminated by a bank of halogen lamps and two 6 kW Xenon arc lamps simulating the solar spectrum (further details can be found in Alfarra et al., 2012, 2013). The air charge in the bag was dried and filtered for gaseous impurities and particles, prior to humidification with high purity de-ionized water. The biogenic SOA precursor α-pinene was injected into the chamber, with an initial mixing ratio of 125 ppb. NOx was added, with an initial mixing ratio of 30 ppb. The relative humidity was 40 %, and the temperature was 25 ∘C.
Gas-phase measurements were made from the chamber through a 0.75 m long PTFE 6.5 mm OD unheated inlet drawn at 2.2 SLM. Particles were collected through a 1.0 m stainless steel 6.2 mm OD inlet at a flow rate of 2 SLM. The same procedure for obtaining the filter background and the same thermal desorption cycle as used in the UMan FIGAERO-CIMS PEG experiments were utilized for the chamber experiments. First, a new filter was placed in the FIGAERO, and the temperature was ramped to 200 ∘C for 10 min to ensure the filter was clean and then cooled. A ramp, soak and cool cycle matching that of the subsequent sample was then completed to obtain the filter background. In addition, and after the filter background, a chamber background was then taken daily that involved a 45 min filter collection of air from the chamber with no VOC added and with no detectable particles in the chamber and subsequent desorption. The chamber experiments were performed using a 45 min trace gas analysis and collection on to the PTFE filter.
2.4 FIGAERO-CIMS for vapour pressure measurements
Previous VP measurements have revealed discrepancies in vapour pressures between instruments that differ between compounds depending on the functional groups they contain. In such previous studies it has not proven straightforward to attribute low or high biases to a particular technique, as shown in the Huisman et al. (2013) study. In the following analysis it is assumed there are no functional group or compound-specific dependencies applicable to the FIGAERO, for the PEG, single-component or unknown compound analysis. This work also makes the necessary assumption that this filter-based measurement in an uncharacterized mixed matrix yields single-component subcooled liquid VPs.
The methodology for retrieving vapour pressures we present in this paper may be subject to some biases when applied to complex chemical systems, and this needs to be borne in mind when interpreting results. When measuring thermograms of multi-component systems collected on the FIGAERO, the desorption profiles can exhibit double and/or non-Gaussian peak shapes, often explained by decomposition of higher molecular weight compounds. The thermal decomposition of higher molecular weight compounds can certainly generate errors in the FIGAERO-CIMS Tmax measurements. This is because any lower molecular weight fragments generated by decomposition will exhibit Tmax values representative of the Tmax of the higher molecular weight molecule from which the fragment was generated. There also may be fragmentation of weak bonds in the particulate phase, also giving an unrepresentative Tmax and desorption profile. A detailed discussion of such factors is given in Stark et al. (2017). Furthermore, inherent to CIMS, whilst the molecular composition can be determined, the molecular structure is not known, and assumptions have to be made based on likely functional groups present in the system (chamber or environment) that is being measured. Recent studies (Booth et al., 2012; Bannan et al., 2017; Dang et al., 2018) have shown how subtle differences in molecular structure have a significant impact upon vapour pressure. Booth et al. (2012), for example, measured the role of ortho, meta and para isomerism in measured solid state and derived subcooled liquid vapour pressures of substituted benzoic acids and observed variations of up to 3 orders of magnitude as a function of this isomerism. Such isomers cannot be differentiated with the CIMS, and therefore the assignment of measured Tmax of compounds with this functional group positioning effect could be dubious and provide broadening or additional peaks, thus affecting the definition of the Tmax and our methodology presented here.
3.1 The relationship between VP and Tmax
Thermograms are shown in Fig. 3 for the PEG samples as measured by the UMan FIGAERO-CIMS, from which the Tmax values are retrieved in a process described above. Tmax values and reported VP literature values for the PEG compounds (Krieger et al., 2018) are summarized in Table 1 and Fig. 4. The vapour pressure range of the PEG series covers an atmospherically relevant range between 1 and 10−7 Pa, where compounds with P298 K>1 Pa exist entirely in the gas phase under atmospherically reasonable conditions, and compounds with Pa will exist largely in the particle phase (Valorso et al., 2011). This range of compounds allows characterization of the FIGAERO across the range of volatilities that are most important throughout the lower atmosphere.
Table 1Reported vapour pressure measurements of the PEG series (4–8) at 298.15 K (Krieger et al., 2018) and associated Tmax values extracted from the UMan FIGAERO-CIMS. All Tmax values are an average of four individual thermograms for each PEG sample. Errors in the Tmax are the maximum variation seen within the four thermograms.
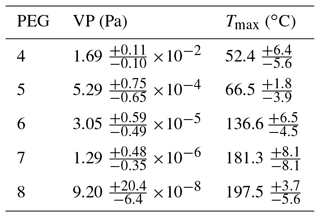
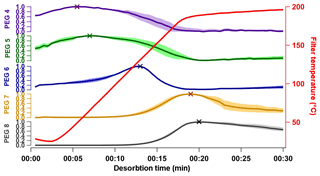
Figure 3Thermograms from the PEG series as detected by the FIGAERO-CIMS employing iodide adduct ionization; all product ion intensities are normalized to 1. Thick coloured lines show the mean of the thermograms, and the associated shaded areas show the standard deviation of the four thermograms. Crosses show the extracted Tmax. The red line shows the temperature just above the filter.
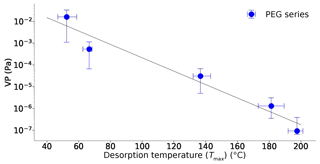
Figure 4Reported vapour pressure measurements of the PEG series (4–8) and associated Tmax values extracted from the UMan FIGAERO-CIMS. Errors on the y axis are those reported in the Krieger et al. (2018) study. Errors in the Tmax (x axis) are the maximum variation seen within the four thermograms from which the mean value was derived.
A single exponential fit to the data on the VP at 298 K derived from the PEG series and extracted Tmax can provide a relationship between Tmax and VP:
3.2 Evaluating the VP calibration for FIGAERO using single compounds with known VP
The PEG VP calibration can be used to derive the VP of other compounds measured by the FIGAERO ToF-CIMS by extracting the Tmax of compounds and applying Eq. (1) to the measured value. By choosing a range of compounds with known and characterized VP, the calibration can be evaluated and may then be utilized for compounds of unknown VP that can be measured with the CIMS.
By way of validation, Table 2 and Fig. 5 show laboratory single-component measurements of Tmax, for a variety of carboxylic acid species, alongside both literature values of their vapour pressure and their calculated vapour pressures using the PEG calibration curve. Whilst these measurements come from both the UMan and GU FIGAERO-CIMS, the PEG series was not measured by the GU FIGAERO-CIMS. Therefore the same calibration function, derived from the UMan CIMS, is utilized for other instruments. Table 3 and Fig. 6 report extracted Tmax and calculated VPs from a chamber experiment in the Manchester Aerosol Photochemical Chamber using the UMan FIGAERO-CIMS. Where possible, the recommended VP values from the Bilde et al. (2015) study are used for comparison, as these are the best available literature values available other than the PEG series.
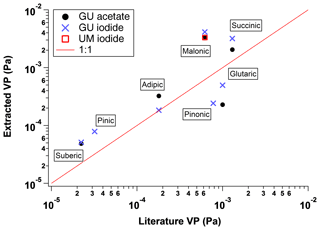
Figure 5Extracted VP from the UMan and GU FIGAERO-CIMS, plotted against reported subcooled saturation vapour pressures from the literature, through utilization of the PEG calibration. These measurements are made using single compounds from the UMan and GU FIGAERO-CIMS (see Table 3).
Table 2Extracted Tmax values and calculated VPs through utilization of the PEG calibration compared against literature data of subcooled saturation vapour pressures. A single-component measurement is defined as a single calibration compound being placed on the filter and desorbed as per the description in the methods.
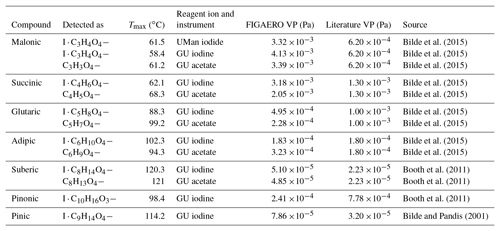
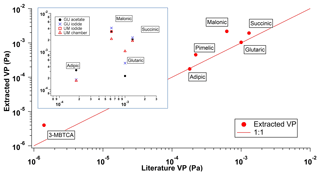
Figure 6Extracted VP from the UMan FIGAERO plotted against reported VPs from the literature. These measurements are made using the UMan instrument from the Manchester SOA chamber experiments (see Table 3). Direct comparisons are made for adipic, glutaric, malonic and succinic acids, which were measured in both the single-component and chamber study, shown on the inset panel. Axes are as described in the main figure. Total mass concentrations in the chamber ranged from 0 to 60 µg m−3 throughout the complete experiment.
Using the PEG series calibration for single-component measurements, it is clear from Table 2 that there is a good agreement between the FIGAERO and literature vapour pressures. The measurements from the chamber also show a good agreement, with an average overestimation of 67 %. This overestimation is well within the reported error of instruments such as the KEMS in the subcooled liquid state (Booth et al., 2012). Figures 5 and 6 show that for the compounds presented by Bilde et al. (2015), as well as selected others, the GU and UMan Tmax-extracted VPs agree very well with the literature. This shows that the PEG series calibration could potentially be applied for different instruments and different reagent ions, depending of course on the configuration and generation of FIGAERO that is being used. Nevertheless, calibration of individual FIGAERO inlets is highly recommended as small changes in the position of the thermocouple, contact time with the heater and nitrogen, nitrogen flow rate and surface area of the filter, among other factors, can affect thermograms.
Table 3Extracted Tmax values and VP at 298 K from chamber SOA experiments as measured by the UMan iodide FIGAERO-CIMS. Tmax values are an average measured over the seven desorptions (not including background) from the chamber experiment. Errors in the Tmax are the maximum variation seen within the seven thermograms. Total mass concentrations in the chamber ranged from 0 to 60 µg m−3 throughout the complete experiment.

We present here the calibration of two FIGAERO inlets coupled to the ToF-CIMS for extracting volatility information from single-component and chamber measurements. Recent comparison of vapour pressure measurement techniques (Krieger et al., 2018) has identified the PEG series as a group of compounds that can be trusted as reference compounds for a range of measurement methods across a wide range of tropospherically representative vapour pressures. This paper shows that this series can be used to calibrate the vapour pressure of single components using the FIGAERO inlet coupled to ToF-CIMS. We have evaluated the derived vapour pressures against a wider range of atmospherically relevant single compounds and compounds identified in chamber oxidation experiments that have a known vapour pressure and demonstrate consistency with other VP techniques. This offers a pathway to determining VPs from FIGAERO-ToF-CIMS for the many atmospheric compounds that are not yet characterized.
We do note that the FIGAERO is not interference-free; mixtures affecting single-component VPs and state differences in mixed component systems will affect retrieved VPs, especially when organic aerosol concentrations are high. Despite the seemingly good agreement with the UMan and GU FIGAERO for the measurements reported here, it is necessary to independently calibrate each FIGAERO inlet, especially when using a generation of FIGAERO different to the commercially available inlet. The authors believe that such single-component measurements of reference compounds, most accurately and confidently using the PEG series, are essential for understanding the extracted information from the FIGAERO, and other VP measurement techniques, in order to better understand the atmospheric implications of such measurements.
The stability of the PEGs allowed sharing of samples to ensure the same quality between the institutions as those that participated in the Krieger et al. (2018) study. Samples should be stored, handled and measured on the same timescale to reduce as much as possible the chance of contamination. We propose that the same procedure could be undertaken to run an inter-comparison between different FIGAERO inlets.
All the data used in this paper are presented in the tables.
The supplement related to this article is available online at: https://doi.org/10.5194/amt-12-1429-2019-supplement.
TJB, MLB, CJP, and DT conceptualized and planned the study and conducted data interpretation. TJB, MLB, MP, SDW, AB, NAM, AM, JH, MH, and MRA conducted data collection and analysis. UKK, JPR, JJ, WR, GM, HC, and CJP provided specific inputs to the paper and Supplement. All co-authors discussed the results and commented on the paper and the Supplement.
The authors declare that they have no conflict of interest.
This work was part funded under NERC grant NE/N013794/1. John Jayne
acknowledges the U.S. Department of Energy Small Business Innovative Research
program (award number DE-SC0004577) that funded the development and
commercialization of the CIMS-FIGAERO system reported here and the supporting
collaboration with Felipe Lopez-Hilfiker and Joel Thornton from the
University of Washington.
Edited by: Bin
Yuan
Reviewed by: three anonymous referees
Alfarra, M. R., Hamilton, J. F., Wyche, K. P., Good, N., Ward, M. W., Carr, T., Barley, M. H., Monks, P. S., Jenkin, M. E., Lewis, A. C., and McFiggans, G. B.: The effect of photochemical ageing and initial precursor concentration on the composition and hygroscopic properties of β-caryophyllene secondary organic aerosol, Atmos. Chem. Phys., 12, 6417–6436, https://doi.org/10.5194/acp-12-6417-2012, 2012.
Alfarra, M. R., Good, N., Wyche, K. P., Hamilton, J. F., Monks, P. S., Lewis, A. C., and McFiggans, G.: Water uptake is independent of the inferred composition of secondary aerosols derived from multiple biogenic VOCs, Atmos. Chem. Phys., 13, 11769–11789, https://doi.org/10.5194/acp-13-11769-2013, 2013.
Bannan, T. J., Booth, A. M., Jones, B. T., O'Meara, S., Barley, M. H., Riipinen, I., Percival, C. J., and Topping, D.: Measured Saturation Vapor Pressures of Phenolic and Nitro-aromatic Compounds, Environ. Sci. Technol., 51, 3922–3928, 2017.
Bilde, M. and Pandis, S. N.: Evaporation rates and vapor pressures of individual aerosol species formed in the atmospheric oxidation of α-and β-pinene, Environ. Sci. Technol., 35, 3344–3349, 2001.
Bilde, M., Svenningsson, B., Mønster, J., and Rosenørn, T.: Even-odd alternation of evaporation rates and vapor pressures of C3− C9 dicarboxylic acid aerosols, Environ. Sci. Technol., 37, 1371–1378, 2003.
Bilde, M., Barsanti, K., Booth, M., Cappa, C. D., Donahue, N. M., Emanuelsson, E. U., McFiggans, G., Krieger, U. K., Marcolli, C., Topping, D., Ziemann, P., Barley, M., Clegg, S., Dennis-Smither, B., Hallquist, M., Hallquist, A. M., Khlystov, A., Kulmala, M., Mogensen, D., Percival, C. J., Pope, F., Reid, J. P., Ribeiro da Silva, M. A. V., Rosenoern, T., Salo, K., Soonsin V. P., Yli-Juuti, T., Prisle, N. L., Pagels, J., Rarey, J., Zardini A. A., and Riipinen I.: Saturation vapor pressures and transition enthalpies of low-volatility organic molecules of atmospheric relevance: from dicarboxylic acids to complex mixtures, Chem. Rev., 115, 4115–4156, 2015.
Booth, A. M., Markus, T., McFiggans, G., Percival, C. J., Mcgillen, M. R., and Topping, D. O.: Design and construction of a simple Knudsen Effusion Mass Spectrometer (KEMS) system for vapour pressure measurements of low volatility organics, Atmos. Meas. Tech., 2, 355–361, https://doi.org/10.5194/amt-2-355-2009, 2009.
Booth, A. M., Montague, W. J., Barley, M. H., Topping, D. O., McFiggans, G., Garforth, A., and Percival, C. J.: Solid state and sub-cooled liquid vapour pressures of cyclic aliphatic dicarboxylic acids, Atmos. Chem. Phys., 11, 655–665, https://doi.org/10.5194/acp-11-655-2011, 2011.
Booth, A. M., Bannan, T., McGillen, M. R., Barley, M. H., Topping, D. O., McFiggans, G., and Percival, C. J.: The role of ortho, meta, para isomerism in measured solid state and derived sub-cooled liquid vapour pressures of substituted benzoic acids, RSC Advances, 2, 4430–4443, 2012.
Booth, A. M., Bannan, T. J., Benyezzar, M., Bacak, A., Alfarra, M. R., Topping, D., and Percival, C. J.: Development of lithium attachment mass spectrometry–knudsen effusion and chemical ionisation mass spectrometry (KEMS, CIMS), Analyst, 142, 3666–3673, 2017.
D'Ambro, E. L., Lee, B. H., Liu, J., Shilling, J. E., Gaston, C. J., Lopez-Hilfiker, F. D., Schobesberger, S., Zaveri, R. A., Mohr, C., Lutz, A., Zhang, Z., Gold, A., Surratt, J. D., Rivera-Rios, J. C., Keutsch, F. N., and Thornton, J. A.: Molecular composition and volatility of isoprene photochemical oxidation secondary organic aerosol under low- and high-NOx conditions, Atmos. Chem. Phys., 17, 159–174, https://doi.org/10.5194/acp-17-159-2017, 2017.
Dang, C., Bannan, T. J., Waters, J., Priestley, M., Percival, C. J., and Topping, D.: Solid state and subcooled liquid vapor pressures of cresols, nitrocresols, methylpyridine carboxylic acids and cycloalkane carboxylic acids as measured with the KEMS, submitted, 2018.
Donahue, N. M., Epstein, S. A., Pandis, S. N., and Robinson, A. L.: A two-dimensional volatility basis set: 1. organic-aerosol mixing thermodynamics, Atmos. Chem. Phys., 11, 3303–3318, https://doi.org/10.5194/acp-11-3303-2011, 2011.
Faxon, C., Hammes, J., Le Breton, M., Pathak, R. K., and Hallquist, M.: Characterization of organic nitrate constituents of secondary organic aerosol (SOA) from nitrate-radical-initiated oxidation of limonene using high-resolution chemical ionization mass spectrometry, Atmos. Chem. Phys., 18, 5467–5481, https://doi.org/10.5194/acp-18-5467-2018, 2018.
Glasius, M. and Goldstein, A. H.: Recent discoveries and future challenges in atmospheric organic chemistry, Environ. Sci. Technol., 50, 2754–2764, 2016.
Huisman, A. J., Krieger, U. K., Zuend, A., Marcolli, C., and Peter, T.: Vapor pressures of substituted polycarboxylic acids are much lower than previously reported, Atmos. Chem. Phys., 13, 6647–6662, https://doi.org/10.5194/acp-13-6647-2013, 2013.
Jimenez, J. L., Canagaratna, M. R., Donahue, N. M., Prevot, A. S. H., Zhang, Q., Kroll, J. H., DeCarlo, P. F., Allan, J. D., Coe, H., Ng, N. L., Aiken, A. C., Docherty, K. S., Ulbrich, I. M., Grieshop, A. P., Robinson, A. L., Duplissy, J., Smith, J. D., Wilson, K. R., Lanz, V. A., Hueglin, C., Sun, Y. L., Tian, J., Laaksonen, A., Raatikainen, T., Rautiainen, J., Vaattovaara, P., Ehn, M., Kulmala, M., Tomlinson, J. M., Collins, D. R., Cubison, M. J., Dunlea, J., Huffman, J. A., Onasch, T. B., Alfarra, M. R., Williams, P. I., Bower, K., Kondo, Y., Schneider, J., Drewnick, S, Borrmann, S., Weimer, S., Demerjian, K., Salcedo, D., Cottrell, L., Griffin, R., Takami, A., Miyoshi, T., Hatakeyama, S., Shimono, A., Sun, J. Y., Zhang, Y. M., Dzepina, K., Kimmel, J. R., Sueper, D., Jayne, J. T., Herndon, S. C., Trimborn, A. M., Williams, L. R., Wood, E. C., Middlebrook, A. M., Kolb, C. E., Baltensperger, U., and Worsnop D. R.: Evolution of organic aerosols in the atmosphere, Science, 326, 1525–1529, 2009.
Junninen, H., Ehn, M., Petäjä, T., Luosujärvi, L., Kotiaho, T., Kostiainen, R., Rohner, U., Gonin, M., Fuhrer, K., Kulmala, M., and Worsnop, D. R.: A high-resolution mass spectrometer to measure atmospheric ion composition, Atmos. Meas. Tech., 3, 1039–1053, https://doi.org/10.5194/amt-3-1039-2010, 2010.
Krieger, U. K., Siegrist, F., Marcolli, C., Emanuelsson, E. U., Gøbel, F. M., Bilde, M., Marsh, A., Reid, J. P., Huisman, A. J., Riipinen, I., Hyttinen, N., Myllys, N., Kurtén, T., Bannan, T., Percival, C. J., and Topping, D.: A reference data set for validating vapor pressure measurement techniques: homologous series of polyethylene glycols, Atmos. Meas. Tech., 11, 49-63, https://doi.org/10.5194/amt-11-49-2018, 2018.
Le Breton, M., Wang, Y., Hallquist, Å. M., Pathak, R. K., Zheng, J., Yang, Y., Shang, D., Glasius, M., Bannan, T. J., Liu, Q., Chan, C. K., Percival, C. J., Zhu, W., Lou, S., Topping, D., Wang, Y., Yu, J., Lu, K., Guo, S., Hu, M., and Hallquist, M.: Online gas- and particle-phase measurements of organosulfates, organosulfonates and nitrooxy organosulfates in Beijing utilizing a FIGAERO ToF-CIMS, Atmos. Chem. Phys., 18, 10355–10371, https://doi.org/10.5194/acp-18-10355-2018, 2018a.
Le Breton, M., Hallquist, Å. M., Pathak, R. K., Simpson, D., Wang, Y., Johansson, J., Zheng, J., Yang, Y., Shang, D., Wang, H., Liu, Q., Chan, C., Wang, T., Bannan, T. J., Priestley, M., Percival, C. J., Shallcross, D. E., Lu, K., Guo, S., Hu, M., and Hallquist, M.: Chlorine oxidation of VOCs at a semi-rural site in Beijing: significant chlorine liberation from ClNO2 and subsequent gas- and particle-phase Cl–VOC production, Atmos. Chem. Phys., 18, 13013–13030, https://doi.org/10.5194/acp-18-13013-2018, 2018b.
Lee, B. H., Lopez-Hilfiker, F. D., Mohr, C., Kurtein, T., Worsnop, D. R., and Thornton, J. A.: An iodide-adduct high-resolution time-of-flight chemical-ionization mass spectrometer: Application to atmospheric inorganic and organic compounds, Environ. Sci. Technol., 48, 6309–6317, 2014.
Lienhard, D. M., Huisman, A. J., Krieger, U. K., Rudich, Y., Marcolli, C., Luo, B. P., Bones, D. L., Reid, J. P., Lambe, A. T., Canagaratna, M. R., Davidovits, P., Onasch, T. B., Worsnop, D. R., Steimer, S. S., Koop, T., and Peter, T.: Viscous organic aerosol particles in the upper troposphere: diffusivity-controlled water uptake and ice nucleation?, Atmos. Chem. Phys., 15, 13599–13613, https://doi.org/10.5194/acp-15-13599-2015, 2015.
Lopez-Hilfiker, F. D., Mohr, C., Ehn, M., Rubach, F., Kleist, E., Wildt, J., Mentel, Th. F., Lutz, A., Hallquist, M., Worsnop, D., and Thornton, J. A.: A novel method for online analysis of gas and particle composition: description and evaluation of a Filter Inlet for Gases and AEROsols (FIGAERO), Atmos. Meas. Tech., 7, 983–1001, https://doi.org/10.5194/amt-7-983-2014, 2014.
Lopez-Hilfiker, F. D., Mohr, C., D'Ambro, E. L., Lutz, A., Riedel, T. P., Gaston, C. J., Iyer, S., Zhang, Z., Gold, A., Surratt, J. D., Lee, B. H., Kurten, T., Hu, W. W., Jimenez, J., Hallquist, M., and Thornton J. A.: Molecular composition and volatility of organic aerosol in the Southeastern US: implications for IEPOX derived SOA, Environ. Sci. Technol., 50, 2200–2209, 2016.
McVay, R. C., Zhang, X., Aumont, B., Valorso, R., Camredon, M., La, Y. S., Wennberg, P. O., and Seinfeld, J. H.: SOA formation from the photooxidation of α-pinene: systematic exploration of the simulation of chamber data, Atmos. Chem. Phys., 16, 2785–2802, https://doi.org/10.5194/acp-16-2785-2016, 2016.
O'Meara, S., Booth, A. M., Barley, M. H., Topping, D., and McFiggans, G.: An assessment of vapour pressure estimation methods, Phys. Chem. Chem. Phys., 16, 19453–19469, 2014.
Priestley, M., Le Breton, M., Bannan, T. J., Leather, K. E., Bacak, A., Reyes-Villegas, E., De Vocht., F., Shallcross, B. M. A., Brazier, T., Khan, M. A., Allan, J., Shallcross, D., E., Coe, H., and Percival, C. J.: Observations of isocyanate, amide, nitrate and nitro compounds from an anthropogenic biomass burning event using a ToF-CIMS, J. Geophys. Res.-Atmos., 123, 7687–7704, 2018a.
Priestley, M., le Breton, M., Bannan, T. J., Worrall, S. D., Bacak, A., Smedley, A. R. D., Reyes-Villegas, E., Mehra, A., Allan, J., Webb, A. R., Shallcross, D. E., Coe, H., and Percival, C. J.: Observations of organic and inorganic chlorinated compounds and their contribution to chlorine radical concentrations in an urban environment in northern Europe during the wintertime, Atmos. Chem. Phys., 18, 13481–13493, https://doi.org/10.5194/acp-18-13481-2018, 2018b.
Reyes Villegas, E., Bannan, T. J., Le Breton, M., Mehra, A., Priestley, M., Percival, C., Allan, J., and Coe, H.: Online chemical characterization of food cooking organic aerosols: implications for source apportionment, Environ. Sci. Technol., 52, 5308–5318, 2018.
Robinson, A. L., Donahue, N. M., Shrivastava, M. K., Weitkamp, E. A., Sage, A. M., Grieshop, A. P., Lane, T. E., Pierce, J. R., and Pandis, S. N.: Rethinking organic aerosols: Semivolatile emissions and photochemical aging, Science, 315, 1259–1262, 2007.
Rovelli, G., Miles, R. E., Reid, J. P., and Clegg, S. L.: Accurate Measurements of Aerosol Hygroscopic Growth over a Wide Range in Relative Humidity, J. Phys. Chem. A, 120, 4376–4388, 2016.
Schobesberger, S., D'Ambro, E. L., Lopez-Hilfiker, F. D., Mohr, C., and Thornton, J. A.: A model framework to retrieve thermodynamic and kinetic properties of organic aerosol from composition-resolved thermal desorption measurements, Atmos. Chem. Phys., 18, 14757–14785, https://doi.org/10.5194/acp-18-14757-2018, 2018.
Soonsin, V., Zardini, A. A., Marcolli, C., Zuend, A., and Krieger, U. K.: The vapor pressures and activities of dicarboxylic acids reconsidered: the impact of the physical state of the aerosol, Atmos. Chem. Phys., 10, 11753–11767, https://doi.org/10.5194/acp-10-11753-2010, 2010.
Stark, H., Yatavelli, R. L., Thompson, S. L., Kang, H., Krechmer, J. E., Kimmel, J. R., Palm B. B., Hu W., Hayes, P. L., Day, D. A., Campuzano-Jost, P., Canagaratna, M. R., Jayne, J. T., Worsnop, D. R., and Jimenez, J. L.: Impact of thermal decomposition on thermal desorption instruments: advantage of thermogram analysis for quantifying volatility distributions of organic species, Environ. Sci. Technol., 51, 8491–8500, 2017.
Topping, D., Reid, J., Rovelli, G., Bannan, T., Dang, C., Laskin, A., Bertram, A., Marcolli, C., Graham, E., Lowe, S., Pope, F., Wilson, K., Krieger, U., Bilde, M., Riipinen, I., Percival C. J., and Petters, M.: International network for coordinating work on the physicochemical properties of molecules and mixtures important for atmospheric particulate matter, EGU General Assembly Conference Abstracts, 20, 18963, 2018.
Tröstl, J., Chuang, W. K., Gordon, H., Heinritzi,, M., Yan, C., Molteni, U., Ahlm, L., Frege, C., Bianchi, F., Wagner, R., Simon, M., Lehtipalo, K., Williamson, C., Craven, J. S., Duplissy, J., Adamov, A., Almeida, J., Bernhammer, A. K., Breitenlechner, M., Brilke, S., Dias, A., Ehrhart, S., Flagan, R. C., Franchin, A., Fuchs, C., Guida, R., Gysel, M., Hansel, A., Hoyle, C. R., Jokinen, T., Junninen, H., Kangasluoma, J., Keskinen, H., Kim, J., Krapf, M., Kürten, A., Laaksonen, A., Lawler, M., Leiminger, M., Mathot, S., Möhler, O., Nieminen, T., Onnela, A., Petäjä, T., Pie,l F. M., Miettinen, P., Rissanen, M. P., Rondo, L., Sarnela, N., Schobesberger, S., Sengupta, K., Sipilä, M., Smith, J. N., Steiner, G., Tomè, A., Virtanen, A., Wagner, A. C., Weingartner, E., Wimmer, D., Winkler, P. M., Ye P., Carslaw, K. S., Curtius, J., Dommen, J., Kirkby, J., Kulmala, M., Riipinen, I., Worsnop, D. R., Donahue, N. M., and Baltensperger U.: The role of low-volatility organic compounds in initial particle growth in the atmosphere, Nature, 533, 527–531, 2016.
Valorso, R., Aumont, B., Camredon, M., Raventos-Duran, T., Mouchel-Vallon, C., Ng, N. L., Seinfeld, J. H., Lee-Taylor, J., and Madronich, S.: Explicit modelling of SOA formation from α-pinene photooxidation: sensitivity to vapour pressure estimation, Atmos. Chem. Phys., 11, 6895–6910, https://doi.org/10.5194/acp-11-6895-2011, 2011.
Zardini, A. A., Krieger, U. K., and Marcolli, C.: White light Mie resonance spectroscopy used to measure very low vapor pressures of substances in aqueous solution aerosol particles, Opt. Express, 14, 6951–6962, 2006.





