the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Development of an antioxidant assay to study oxidative potential of airborne particulate matter
Pourya Shahpoury
Tom Harner
Gerhard Lammel
Steven Lelieveld
Haijie Tong
Jake Wilson
Oxidative potential is a measure of redox activity of airborne particulate matter (PM) and is often used as a surrogate to estimate one form of PM toxicity. The evaluation of oxidative potential in a physiologically relevant environment is always challenging. In this work, we developed a chromatographic method, employing an ultra-high-performance liquid chromatograph coupled to a triple–quadruple mass spectrometer, to determine the oxidative potential of PM from different sources. To this purpose, we measured the PM-induced oxidation of glutathione, cysteine, and ascorbic acid, and formation of glutathione disulfide and cystine, following PM addition to simulated epithelial lining fluids, which, in addition to the antioxidants, contained inorganic salts, a phospholipid, and proteins. The new method showed high precision and, when applied to standard reference PM, the oxidative potential was found to increase with the reaction time and PM concentration in the lung fluid. The antioxidant depletion rates were considerably higher than the rates found with the conventional dithiothreitol assay, indicating the higher sensitivity of the new method. The presence of the lung fluid inorganic species increased the oxidative potential determined through glutathione and cysteine, but showed an opposite effect with ascorbic acid, whereas the presence of proteins resulted in a moderate decrease in the oxidative potential. In the presence of PM2.5, glutathione and cysteine demonstrated similar depletion patterns, which were noticeably different from that of ascorbic acid, suggesting that cysteine could be used as an alternative to glutathione for probing oxidative potential.
- Article
(985 KB) - Full-text XML
-
Supplement
(286 KB) - BibTeX
- EndNote
Air pollution is a major environmental health risk. It has been associated with respiratory and cardiovascular diseases as well as increased morbidity and mortality rates over the past 25 years. Human exposure to fine particulate matter (aerodynamic diameter ≤2.5 µm, i.e. PM2.5) is one of the main causes of the adverse effects across the world (WHO, 2016; Cohen et al., 2017; Burnett et al., 2018; Lelieveld et al., 2018, 2019a). Recent studies suggest that exposure to PM2.5 significantly reduces the human life expectancy in both low-middle income and developed countries (Lelieveld et al., 2018, 2019a), with fossil-fuel-related emission being responsible for 65 % of premature deaths from anthropogenic air pollutants worldwide (Lelieveld et al., 2019b).
For decades, PM mass concentration has been applied as a metric to relate air pollution to adverse health outcomes. However, a large part of PM is made of chemicals with low toxicity, e.g. sea salt, ammonium sulfates, and nitrates, and only a small portion of PM including the organic phase and metals is expected to pose toxic effects (Ayres et al., 2008; Lodovici and Bigagli, 2011). Moreover, the pool of chemicals in PM dynamically changes as it undergoes temporal and spatial evolutions due to reaction with oxidants and mixing in the atmosphere (Pöschl and Shiraiwa, 2015; Paula et al., 2016). Growing evidence links the adverse health effects of air pollution to pulmonary oxidative stress due to increased exposure to atmospheric oxidants or decreased antioxidant defense levels (Kelly, 2003; Li et al., 2003; Künzli et al., 2006; Borm et al., 2007; Weichenthal et al., 2013; Kelly and Fussell, 2017; Bates et al., 2019). Consequently, the oxidative potential of PM2.5 has been applied as an additional parameter to PM mass in explaining the air pollution health effects (Weichenthal et al., 2019).
In the context of inhalation toxicity, oxidative potential is defined as the ability of PM-bound chemicals to oxidize the lung antioxidants and catalytically generate reactive oxygen species, e.g. hydrogen peroxide, superoxide radical, and hydroxyl radical (Bates et al., 2019). Through this process, the redox-active constituents of PM, e.g. quinones and transition metals, react with the antioxidants in the epithelial lining fluid (ELF), resulting in the depletion of antioxidants and formation of oxidation products. In the case of thiol-containing antioxidants, such as glutathione (GSH) and cysteine (CSH), their oxidation leads to formation of glutathione disulfide (GSSG) and cystine (CSSC) through formation of a disulfide bridge (Fig. 1). Although quinones and transition metals have been recognized as important redox-active substances, other redox-active chemicals, such as humic-like substances, organic hydroperoxides, highly oxygenated molecules, and environmentally persistent free radicals, were also found to have oxidative potential (Charrier and Anastasio, 2012; Verma et al., 2015; Dou et al., 2015; Tong et al., 2016, 2017, 2019; Gonzalez et al., 2017).
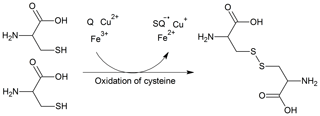
Figure 1Oxidation of CSH through reaction with quinones (Q) and transition metals (Fe3+, Cu2+) and subsequent formation of CSSC, semi-quinones (SQ), and reduced transition metals (Fe2+, Cu+).
To this date, oxidative potential has been primarily determined using colorimetric methods, and the dithiothreitol assay has been used most frequently (Charrier and Anastasio, 2012; Charrier et al., 2014; Verma et al., 2009, 2014, 2015; Calas et al., 2017, 2018; Crobeddu et al., 2017; Tong et al., 2018, Tuet et al., 2019; Bates et al., 2019). Such methods introduce certain challenges; for instance, dithiothreitol is a chemical that is alien to the ELF, and the assay was shown to have a greater response to PM-bound quinones rather than metals (Ayres et al., 2008; Charrier et al., 2014); metals outweigh quinones in ambient PM. It has been suggested that the dithiothreitol oxidation rates may not correspond entirely to the formation rates of reactive oxygen species (Xiong et al., 2017; Fang et al., 2019). Moreover, dithiothreitol oxidation demonstrates degrees of association to changes in PM components, sources (e.g. biomass burning, traffic), and temporal variations that are different from those seen with GSH or ascorbic acid (AA) (Fang et al., 2016; Weichenthal et al., 2019). Such observations raise the question of whether the dithiothreitol oxidation rate is a realistic metric for oxidative potential of ambient PM in physiological ELF.
Colorimetric methods are not fully compatible with the ELF containing lipids and proteins because the turbidity resulting from their presence in ELF could interfere with the reading of a UV probe. Extraction of PM in simulated ELF (SELF), as opposed to water, as well as SELF formulation could in various ways affect the oxidative potential results (Calas et al., 2017). The presence of lipids and proteins could be particularly important as they influence the ELF characteristics: for instance, dipalmitoylphosphatidylcholine (DPPC), a major ELF surfactant phospholipid, is known to reduce the ELF surface tension (Gregory et al., 1991; Griese, 1999; Johansson et al., 1994; Pison et al., 1986; Boisa et al., 2014). The presence of DPPC has been linked to improved oxidative potential dose-response relationships, supposedly by enhancing suspension stability (Calas et al., 2017). Albumin, a major ELF protein (Bredberg et al., 2012), binds to metals and limits their solubility, hence influencing ELF antioxidant and PM pro-oxidant capacity (Cross et al., 1994).
Chromatographic methods have been applied less frequently to studies of oxidative potential from air samples (Mudway and Kelly, 1998; Zielinski et al., 1999; Künzli et al., 2006; Crobeddu et al., 2017; Calas et al., 2018). These studies determined the consumption of AA in a simplified SELF (0.9 % saline, pH 7.4) using a high-performance liquid chromatograph coupled with electrochemical detection (Iriyama et al., 1984). Crobeddu et al. (2017) advanced this method by measuring GSH and GSSG in addition to AA; however, they did not take into account potential artifacts due to oxidation of GSH during sample processing. This effect was shown to be a major source of uncertainty in measuring thiol-containing antioxidants in biological samples (Giustarini et al., 2016). The artifact can happen during sample processing due to various factors, such as sample acidification or restoration of pH to neutral/alkaline (Rossi et al., 2002), which may lead to variable oxidation of thiols and low reproducibility of measured GSH and GSSG concentrations.
In the present work, a new chromatographic method has been developed to determine the oxidative potential of ambient PM using an ultra-high-performance liquid chromatograph (UHPLC) coupled to a triple–quadruple mass spectrometer (MS/MS). This method measures the direct consumption of AA, CSH, and GSH and formation of CSSC and GSSG following incubation of PM in SELF which, in addition to antioxidants, contains major ELF inorganic salts, a phospholipid, and proteins. The use of UHPLC-MS/MS allows a relatively short analytical time down to a few minutes, whereas the MS/MS capability allows simultaneous detection of multiple analytes. This is a major advantage compared to conventional HPLC analysis with absorbance detection, and it is important when performing high-throughput laboratory analysis, in particular when analyzing high-molecular mass substances with various organic or water solubility. Pre-analytical sample preparation is key to achieving optimal results in the analysis of antioxidants. In the case of GSH and CSH, masking the –SH group is crucial in preventing their artificial oxidation prior to instrumental analysis (Giustarini et al., 2016; Escobar et al., 2016). In the present study, this has been achieved by derivatizing GSH and CSH with N-ethylmaleimide (NEM), which is an effective alkylating reagent for masking the –SH group at the pH range of 6.5–7.5. We investigated the method performance and reproducibility, the oxidative potential time dependence and dose response, and the effect of SELF composition on PM oxidative potential. We compared the current method with the dithiothreitol assay and applied the method to contemporary PM2.5 samples.
2.1 Chemicals
AA, GSH, GSSG, and isotopically labelled AA–13C6, GSH–, CSH–13C3, and GSSG– were purchased from Toronto Research Chemicals (Toronto, Canada). CSH, CSSC, NEM, DPPC, ethylenediaminetetraacetic acid (EDTA), sulfosalicylic acid (SSA), nitric acid, phosphate-buffered saline (PBS; Bio-Performance, containing NaCl, KCl, and Na2HPO4), calcium chloride (CaCl2), magnesium chloride (MgCl2), sodium sulfate (Na2SO4), glycine, uric acid (UA), and albumin were purchased from Sigma Aldrich (Oakville, Canada). CSSC-d6 was purchased from TLC Pharmaceutical Standards (Aurora, Canada). LC-MS grade acetonitrile and methanol were obtained from Caledon Laboratory Chemicals (Georgetown, Canada), and LC-MS grade (Optima) water and formic acid were purchased from Fisher Scientific (Ottawa, Canada). The stock solution of analytical standards AA, AA–13C6, GSH, GSH–, CSH, CSH–13C3, GSSG, and GSSG– were prepared in 50:50 methanol–H2O mixture, whereas CSSC and CSSC-d6 were prepared in 10:90 methanol–H2O; these were used only for preparing calibration standards. In addition, a 50 mM mixture of AA, GSH, and CSH was prepared in 20:80 methanol–H2O and used specifically to prepare SELF. All antioxidant stock solutions were stored at −80 ∘C. The 20 % methanol solution allowed swift thawing (∼1 min) of concentrated antioxidant mixture at room temperature and helped prevent oxidation of antioxidants due to long thawing times prior to the experiments; 100 mM stock solution of NEM and a solution containing EDTA (2 mM) and SSA (2 %) were prepared in 20:80 methanol–H2O and stored at 4 ∘C. We avoided glass-ware for sample processing, and all plastic-ware used in this study were cleaned with Nalgene L900 soap, 0.1 M nitric acid, deionized water, and LC-MS grade methanol.
2.2 Sample characteristics
2.2.1 Particulate matter
Standard reference material (SRM 1649b) was obtained from the National Institute of Standard and Technology (NIST, Gaithersburg, USA). The sample is characterized for a large number of organic and inorganic species, including some redox-active substances, i.e. 9,10-anthraquinone (1.8 pg µg−1), 1,2-benzanthraquinone (3.6 pg µg−1), copper (311 pg µg−1), and manganese (337 pg µg−1). In terms of mass-size distribution, the volume-weighed mean falls in the range of coarse atmospheric particles. In the present study, SRM suspension in LC-MS grade water was freshly made (5 mg mL−1) and used across different experiments. The PM suspension was gently mixed each time prior to spiking into SELF. In addition, PM2.5 samples were obtained from the National Air Pollution Surveillance (NAPS) site in Hamilton, Canada, and used for validating the antioxidant assay. The samples were collected on quartz fiber filters using a Dichotomous Air Sampler (Thermo Scientific, Waltham, USA), each for a period of 24 h. The sampled air volume was 21.6 m3 in each case, and a 1 cm2 filter punch from each sample was used to perform the antioxidant assay (this denotes 1.6 m3 of the sampled air for each sample). The PM2.5 concentrations for these samples ranged between 14.3 and 16.1 µg m−3. Lab blanks consisted of quartz fiber filters that were not used for air sampling.
Table 1Compositions of SELF used in this study.
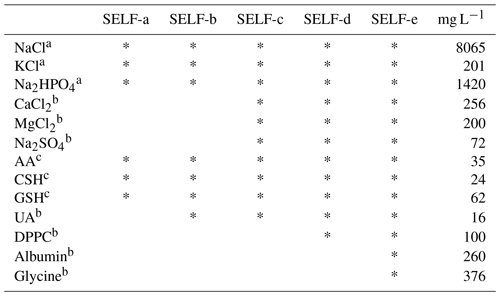
a Composition of standard PBS used (pH 7.4). b Follows reported physiological concentrations (Boisa et al., 2014). c 200 µM of each antioxidant was used; SELF: simulated epithelial lining fluid, AA: ascorbic acid, CSH: cysteine, GSH: glutathione, UA: uric acid, DPPC: dipalmitoylphosphatidylcholine.
2.2.2 SELF formulations
Different SELF formulations representing extracellular fluid of human lung were tested in the present study; a simplified formula was used as the basis for all formulations and contained PBS (pH 7.4) and a mixture of CSH, GSH, and AA (200 µM each; Table 1). These salts and antioxidants are among the constituents of a Gamble solution and physiological ELF (Stopford et al., 2003; Boisa et al., 2014). Four other formulations were used in order to explore the effects of SELF composition on oxidative potential results. These formulas, in addition to the above components, contained UA, CaCl2, MgCl2, Na2SO4, DPPC, albumin, and glycine at physiological concentrations (Boisa et al., 2014). The presence of inorganic salts, antioxidants, surfactant lipids, and proteins could affect the dissolution kinetics of redox-active species (Boisa et al., 2014). The pH of SELF formulations was monitored during incubation in an independent experiment.
2.3 Sample preparation
The stock solution of reduced antioxidants (50 mM) was swiftly thawed (∼1 min) at room temperature before each experiment and spiked into SELF to achieve antioxidant concentrations of 200 µM each using a positive displacement pipette (Microman E, Gilson, Middleton, USA); 2.5 mL of SELF was transferred to pre-cleaned 8 mL low-density polyethylene (LDPE) bottles (Nalgene, Thermo Scientific, Waltham, USA). LDPE bottles containing SELF were spiked with PM stock suspension in order to achieve PM concentrations ranging from 10 to 80 µg mL−1. For experiments involving PM2.5 samples from the NAPS site, a 1 cm2 filter punch was added to SELF. The samples containing PM were gently mixed and incubated along with reference SELF that did not contain PM. The incubation was performed at 37 ∘C and 60 rpm using an incubator shaker (Benchmark Scientific, Sayreville, USA) at intervals ranging from 30 to 240 min. Following the incubation, 300 µL aliquots (n=3) of each sample were transferred to 1.5 mL centrifuge tubes (Brand, Wertheim, Germany), added with 100 µL of 100 mM NEM, mixed for 5 s using a vortex mixer, and allowed to react at room temperature for 1 min. This reaction time was found to be sufficient for derivatization of thiols (Escobar et al., 2016). The sample was subsequently added with 200 µL of a mixture containing 2 % SSA and 2 mM EDTA (Moore et al., 2013), mixed for 10 s, and centrifuged (10 000 g, 6 min). The SSA in the precipitation mixture was used to precipitate albumin and to reduce the pH to ∼2, whereas EDTA was used to chelate transition metals (Moore et al., 2013). Finally, 5 µL of the supernatant from each sample was transferred to a push-filter vial with a polytetrafluoroethylene filter (Whatman, Pittsburgh, USA) containing 485 µL of 20:80 methanol–H2O and spiked with 10 µL of labelled antioxidant standard mixture (10 ng µL−1). We applied this mixture across all SELF formulations in order to ensure consistency in sample processing. Moreover, we compared our method with the dithiothreitol assay in terms of dose response and reproducibility. The dithiothreitol assay was performed following the procedure described in Tong et al. (2018); more details can be found in Sect. S1 in the Supplement.
2.4 Sample and data analysis
The sample analysis was carried out using an Acquity UHPLC (Waters, Milford, USA) coupled to a Xevo TQ-S MS/MS (Waters). The analysis was performed in electrospray ionization in the negative mode for AA and AA–13C6 and in the positive mode for the rest of the analytes. The auto-sampler temperature was set to 4 ∘C and the injection volume was 2 µL. The target substances were separated using an Acquity HSS T3 UPLC column (3 mm, 50 mm, 1.8 µm; Waters), connected to a pre-column (2.1 mm, 5 mm, 1.8 µm, Waters), and thermostated at 40 ∘C. The mobile phase A was composed of LC-MS grade H2O containing 0.1 % formic acid, and mobile phase B was made of LC-MS grade acetonitrile containing 0.1 % formic acid. The mobile phase flow rate was set to 0.4 mL min−1. For the target compounds in the negative mode, the mobile phase gradient started at 20 % phase B, ramped to 100 % B from 0.3 to 0.5 min and held for 4.5 min, and then decreased to 20 % B from 5 to 5.1 min and held for 2.9 min. For substances in the positive mode, the gradient started at 1 % phase B, ramped to 50 % B from 0.1 to 0.3 min and held for 3.2 min, followed by an increase to 100 % B over 0.1 min and a hold time of 2.4 min, and followed by a decrease to 1 % B and a re-equilibration time of 1.9 min.
Table 2LC-MS/MS detection parameters for target substances.
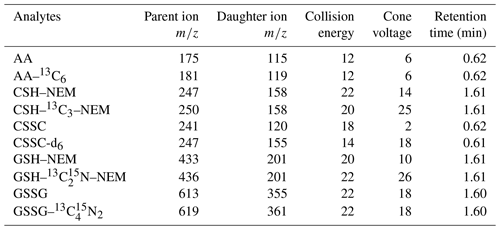
AA: ascorbic acid, CSH: cysteine, CSSC: cystine, GSH: glutathione, GSSG: glutathione disulfide, NEM: N-ethylmaleimide.
The target compound detection parameters are presented in Table 2. The following MS/MS parameters were used: capillary voltage of 1.5 kV, source temperature of 120 ∘C, nitrogen and desolvation gas flow rates of 150 and 900 L h−1, desolvation temperature of 500 ∘C, and collision gas flow rate of 0.15 mL min−1. The analyte quantification was done using the internal calibration method with six-point linear calibration curves (r2=0.999) ranging from 1 to 250 pg µL−1. The chromatographic data analysis was performed using MassLynx software (Waters). Blanks were analyzed after the samples and highest-level calibration standard, and analyte carry-over was below the instrument detection limits (n=3).
Antioxidant consumption (µM min−1) was calculated as follows: (CSHREF–CSHPM)/(incubation time), where CSHREF and CSHPM are the measured concentrations (µM) of CSH in the reference SELF and SELF containing PM following the incubation (min), respectively. The consumptions of AA and GSH were also calculated using the formula above. The oxidation product formation (µM min−1) was calculated as follows: (CSSCPM–CSSCREF)/(incubation time), where CSSCPM and CSSCREF denote the measured concentrations of CSSC in SELF containing PM and the reference SELF after the incubation, respectively. The formation of GSSG was calculated using the same formula.
Availability of the concentrations of redox couples CSH–CSSC and GSH–GSSG allows calculation of the redox state of SELF before and after addition of PM. In the biochemical literature, the Nernst equation has been frequently used to calculate the reduction potential (Eh, expressed in mV) of antioxidant redox couples such as GSH–GSSG (Schafer and Buettner, 2001). Despite some debate about the interpretation of the reduction potentials (Flohé, 2013), in terms of the presence or absence of equilibrium between the redox couples, such values have been used as the measure of oxidation state within biological systems (Jones et al., 2000; Iyer et al., 2009). The molar ratio of the redox couples, e.g. GSH–GSSG, is another metric that has been used widely to determine the oxidative stress in organisms (Giustarini et al., 2016). Both metrics would provide a meaningful way to interpret oxidative potential data, because they consider redox couples as opposed to a single antioxidant; however, one limitation with using ratios is that they could vary from zero to infinity. In this work, in addition to presenting the results as molar concentrations, we calculated the theoretical redox state of CSH–CSSC and GSH–GSSG redox couples, Eh (mV), using the molar concentrations (mol L−1) of these species (Table S1) measured with the present method and following the Nernst equation (at pH 7.4), Eqs. (1) and (2) (Jones et al., 2000; Iyer et al., 2009):
3.1 Method performance
The method reproducibility was tested using SELF-a (Table 1). Four samples containing PM (20 µg mL−1) were incubated together with a reference sample for the duration of 180 min. Three replicates were prepared from each SELF sample containing PM and five replicates prepared from the reference SELF. The mean consumptions for AA, CSH, and GSH, due to the presence of PM, were 0.29±0.03, 0.43±0.03, and 0.53±0.04 µM min−1, respectively, with a relative standard deviation (%RSD) of ≤9 % (Fig. 2). These values reflect the inter-sample reproducibility, targeting the entire sample preparation procedure. The intra-sample variabilities (n=3) were smaller, with %RSD ≤3 %. The formations of CSSC and GSSG were similarly reproducible, i.e. 0.05±0.01 and 0.09±0.01 µM min−1, with inter- and intra-sample RSDs of ≤13 and ≤6 %, respectively (Fig. 2). The results indicate noticeable enhancement in precision compared to those reported by Crobeddu et al. (2017), where derivatization of the –SH group was not considered during sample processing and RSD values of > 50 % were observed in several cases. Re-calculating our results following the Crobeddu et al. (2017) approach (i.e. % depletion), the RSD for GSH depletion with our method would be 8 % for inter-sample variability and ≤5 % for intra-sample variability. High reproducibility is essential for statistically valid comparison of oxidative potential between different samples. Our results further confirm the need for derivatization when analyzing thiol-antioxidants (Giustarini et al., 2016).
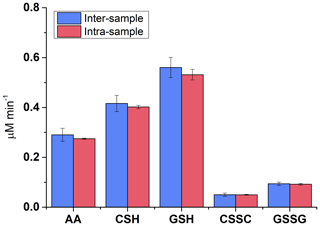
Figure 2Depletion of antioxidants, AA, CSH, and GSH, and formation of oxidation products CSSC and GSSG in SELF-a containing 20 µg mL−1 of SRM and following an incubation time of 180 min. Inter-sample reflects the reproducibility of the entire method (n=4), whereas intra-sample shows the post-incubation variability due to sample processing (from replicates of a single sample; n=3).
We investigated whether our post-derivatization sample processing influenced the oxidative potential results: in a separate experiment, which was conducted with PM in SELF-a, we replaced the precipitation solution (i.e. 2 mM EDTA/2 % SSA) with (a) H2O and (b) 2 mM EDTA. Note that treatment with EDTA/SSA is necessary for effective deposition of proteins prior to instrumental analysis (Moore et al., 2013), when these are present in SELF formulation. The oxidative potential was found to be independent of the processing methods, mainly due to the use of internal standards in our method, which accounted for post-incubation changes in concentrations of target compounds in both reference SELF and SELF containing PM. Among the three treatments, RSDs of the antioxidant depletion and oxidation product formation rates were ≤3 for CSH and GSH, 6 % for AA, and ≤7 % for CSSC and GSSG. The addition of EDTA–SSA mixture, however, prevented the absolute loss of AA by ≤20 % in reference SELF and SELF containing PM, compared to the other two sample treatments. This indicates that the addition of EDTA–SSA helps stabilize AA. There was no considerable difference in the absolute abundance of the other target analytes, which is reasonable given that CSH and GSH underwent derivatization immediately following the incubation. In order to ensure the stability of pH in the SELF mixtures, in a separate experiment, we measured the pH in all reference SELF mixtures and SELF mixtures containing PM (Table 1) over 180 min reaction time. The pH was found to be 7.3±0.1; hence, no noticeable effect of pH on the oxidative potential results could be anticipated.
3.2 Assay kinetics
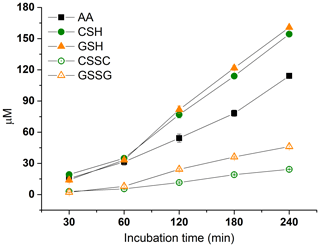
The assay reaction kinetics were studied using SELF-a at a PM concentration of 25 µg mL−1 (Fig. 3). The consumptions of antioxidants were similar in the first 60 min (33±2 µM; n=3); however, beyond this time point, CSH and GSH diverged from AA at increasing rates, and this trend continued with the passing of time (Fig. 3). CSH and GSH showed similar consumptions that were constantly higher than those with AA, with a difference ranging between 3 and 47 µM. The highest consumption rates were found at the last time step; these were 161±1 for GSH, 154±1 for CSH, and 114±1 µM for AA (Fig. 3).
As was seen with the consumption rates of CSH and GSH, the formations of CSSC and GSSG were similar up to a 60 min time step, i.e. ≤3 µM, after which they grew apart at increasing rates. The highest formations were seen at a 240 min interval and were 24±0 and 46±1 µM, respectively (Fig. 3). Considering the molar concentrations of the redox pairs CSSC–CSH and GSSG–GSH, the observed formations of CSSC and GSSG were found to be noticeably lower than the theoretical values anticipated from reaction stoichiometry, i.e. 2 CSH →1 CSSC and 2 GSH →1 GSSG. This was more pronounced for the CSH–CSSC (i.e. 6→1) than GSH–GSSG couple (i.e. 3→1). Such observations could be related to the formation of complexes between the organic molecules in PM and deprotonated –SH prior to formation of CSSC or GSSG, such as glutathionylated quinone species (Song and Buettner, 2010).
3.3 Effect of PM concentration
The response characteristics of the assay to various doses of PM were studied using SELF-a (Table 1), a PM concentration ranging from 10 to 80 µg mL−1, and an incubation time of 180 min. As shown in Fig. 4, the oxidative potential showed an increasing trend with PM concentration. GSH demonstrated the highest consumption, followed by CSH, and with a relatively larger gap by AA. The highest increase in oxidative potential was between PM concentrations of 10 and 40 µg mL−1, and this was relatively more pronounced for CSH and GSH (from 0.32 to 1 µM min−1) than AA (from 0.17 to 0.55 µM min−1). The CSH and GSH depletion rates were linear in the PM concentration of 10–40 µg mL−1 and slowed down beyond this point; the two analytes were found completely consumed at a PM concentration of ≥60 µg mL−1 (Fig. 4). Similar response patterns of CSH and GSH to PM suggest that CSH could be alternatively used to study the oxidative potential of ambient PM; this has not been considered in the past. The AA depletion covered a larger linear range, i.e. up to a PM concentration of 60 µg mL−1, but it slowed down beyond this concentration, with the highest depletion (0.80 µM min−1) found at 80 µg mL−1 PM concentration (Fig. 4). The linear response range of antioxidants could depend on variables such as PM composition and mass-size distribution. AA was shown to respond to both Fe and Cu, along with quinones, whereas GSH had a negligible response to Fe (Ayres et al., 2008).
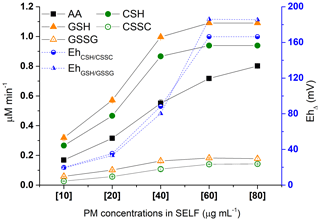
Figure 4Dose response of antioxidants AA, CSH, and GSH depletion and oxidation products CSSC and GSSG formation due to increase in SRM concentration in SELF-a (left y axis) following 180 min incubation; corresponding changes in the redox state of SELF calculated using the Nernst equation (Eh; right y axis), where Δ denotes the difference in Eh between SELF containing PM and reference SELF.
Similarly to antioxidants, the formation of CSSC and GSSG increased with PM concentration (Fig. 4) from 10 to 60 µg mL−1 and plateaued beyond this range. This plateau is explained by the complete loss of CSH and GSH at a PM concentration of ≥60 µg mL−1. The CSSC and GSSG formations were linear up to 40 µg mL−1 PM concentration, in agreement with the depletion values seen for CSH and GSH.
We estimated the theoretical redox state of CSH–CSSC and GSH–GSSG redox pairs (Eh) using the Nernst equation (Eqs. 1 and 2 in Sect. 2.4; Jones et al., 2000; Iyer et al., 2009). The results are presented in Fig. 4 as EhΔ (i.e. EhPM – EhREF) for consistency with the calculation and presentation of antioxidant depletion rates. As shown in the figure, EhΔ increased with PM concentration and oxidative potential, and the overall patterns followed those of CSH and GSH depletion. This demonstrates that the oxidative potential of ambient PM can be presented as Eh, which may be more meaningful because Eh represents a redox couple as opposed to a single antioxidant.
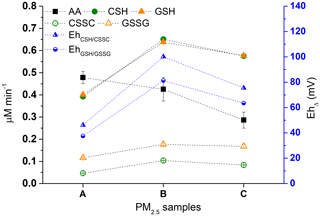
3.3.1 Method application to PM2.5
In order to validate the current method with contemporary ambient samples, we applied the method to PM2.5 samples collected from the NAPS station in Hamilton (Fig. 5, x axis A–C). The samples were incubated in SELF-a for the duration of 180 min. As shown in the figure, the AA, CSH, and GSH mean consumption rates were 0.29-0.48 (RSD: ≤13 %), 0.39-0.65 (RSD: ≤1 %), and 0.40–0.64 (RSD: ≤2 %) µM min−1, respectively. The CSSC and GSSG formation rates were 0.05–0.10 (RSD: ≤3 %) and 0.12–0.18 (RSD: ≤5 %) µM min−1 (Fig. 5). The EhΔ calculated for CSH–CSSC and GSH–GSSG redox couples with the PM2.5 samples were 46–100 and 37–81 mV; their patterns followed closely those of CSH and GSH depletion (Fig. 5), as we have seen with the results of the standard reference material in Sect. 3.3. Similarly, CSSC and GSSG formations were lower than anticipated given the reaction stoichiometry, as we also found with the kinetic study (Sect. 3.2), with CSH–CSSC and GSH–GSSG molar ratios of 7.2±1.1 and 3.5±0.1, respectively. AA did not follow the CSH and GSH depletion patterns, and consequently the EhΔ (Fig. 5), reaffirming that AA and thiol antioxidants respond to different PM constituents and emission sources (Ayres et al., 2008; Fang et al., 2016; Weichenthal et al., 2019).
3.4 Influence of SELF composition
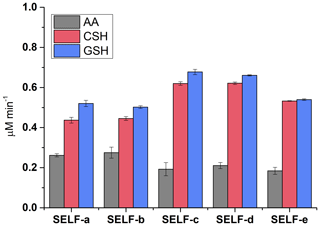
The effect of SELF composition on oxidative potential was examined using SELF formulations that are listed in Table 1. As shown in Figs. 6 and S1a–b, the addition of UA (SELF-b) did not noticeably affect the depletion of antioxidants or formation of CSSC; however, the mean formation rate of GSSG was found to be 0.05 µM min−1 higher in SELF-b than SELF-a. Regardless, this did not result in a difference of EhΔ (Fig. S1b). The presence of additional inorganic salts, CaCl2, MgCl2, and Na2SO4 (SELF-c), led to an increase in the depletion of CSH and GSH by ≤0.18 µM min−1 (Fig. 6) and formation of CSSC by 0.03 µM min−1 (Fig. S1a), and this change resulted in a 13 mV increase in the EhΔ (Fig. S1b). This observation may be related to enhanced electron circulation in the redox system due to the presence of additional electrolytes, or to an increase in the SELF ionic strength, enhancing the solubility of PM-bound transition metals. Interestingly, AA showed an inverse response to this change in SELF formulation, and its depletion was 0.09 µM min−1 smaller in SELF-c than in SELF-b. Further changes in AA depletion due to variation in SELF composition were not observed (Fig. 6). In contrast, the addition of ELF surfactant lipid DPPC (SELF-d) did not result in a noticeable change in OP (Figs. 6 and S1a–b), whereas we observed a small decrease in oxidative potential with the addition of albumin and glycine (SELF-e; ≤0.12 µM min−1 decrease in CSH and GSH depletion and ≤6 mV decrease in EhΔ). The addition of DPPC, however, led to slightly better reproducibility of antioxidant depletion rates (Fig. 6), which is consistent with previous reports (Calas et al., 2017). Overall, our results show that, considering the characteristics of PM used in the present study and with the exception of AA, the oxidative potential that is measured through depletion of antioxidants can be ≤28 % higher in the presence of a SELF which contains additional inorganic salts and lipid or ≤13 % lower following the addition of proteins. This suggests that, in order to determine a more realistic oxidative potential, a SELF that better represents the true human ELF should be used, rather than the simplified formulas that have been used in the past.
3.5 Comparison with the dithiothreitol assay
In order to evaluate the performance of the current method, we tested the SRM sample with the dithiothreitol assay and concentrations of 25 to 100 µg mL−1. The method details and results are presented in the Supplement (Sect. S1 and Fig. S2). As can be seen in Fig. S2, the dithiothreitol assay showed a positive near-linear dose-response relationship, similar to our observation with the antioxidant assay. The mean dithiothreitol consumption ranged from 0.02 to 0.06 µM min−1. Evidently, the consumption rates were considerably lower (nearly an order of magnitude) than the rates found for antioxidants AA, CSH, and GSH, which were determined with the chromatographic method. This difference may be related to the relatively low response of the dithiothreitol assay to the transition metals present in the PM, as previously reported (Ayres et al., 2008; Charrier et al., 2014). Moreover, the dithiothreitol results showed relatively lower reproducibility (RSD: ≤45 %) at the studied PM concentration range when compared to antioxidant assay data. This is not surprising considering the previous results from the dithiothreitol assay in the literature (Charrier and Anastasio, 2012; Fang et al., 2016; Crobeddu et al., 2017). Additional work is being conducted to compare the reaction kinetics between the two assays.
In summary, we developed a method that uses UHPLC-MS/MS to measure the oxidative potential of atmospheric PM. As new features, we included a CSH–CSSC redox pair, involved physiologically relevant ELF components, and explored the SELF redox state (Eh) following the addition of PM. The developed method showed high reproducibility of the measured oxidative potential, both inter- and intra-sample, when compared to previous methods, highlighting the importance of derivatization when analyzing thiol-antioxidants. The depletion rates of antioxidants, the formation rates of oxidation products, and, consequently, the Eh increased with PM concentration in SELF. The method was further validated using contemporary PM2.5 samples, and the results reaffirmed that CSH and GSH have similar depletion rates and patterns, which are different from those found with AA. This similarity suggests that CSH and its redox couple CSSC can be used as alternative target chemicals when determining the oxidative potential of ambient PM. One advantage of CSH–CSSC is that their labelled standards are more readily available. We found that the dithiothreitol response to PM could be up to an order of magnitude lower than the responses seen with the antioxidant assay. This study showed that the presence of additional electrolytes could influence the measured oxidative potential in various ways, increasing the oxidation of thiol-containing antioxidants and decreasing that for AA, whereas proteins may result in a moderate decrease in antioxidant depletion in the presence of PM. Further work needs to be done to realize the potential of this method by including samples that represent the chemical composition of various types of atmospheric aerosols and their spatial and temporal variations.
The dataset used in this paper is available from the corresponding author (pourya.shahpoury@canada.ca).
The supplement related to this article is available online at: https://doi.org/10.5194/amt-12-6529-2019-supplement.
PS developed the antioxidant assay and carried out the chemical and data analysis, SL and HT performed the DTT assay, JW and GL contributed to the discussions on redox potential, and PS wrote the manuscript with contributions from JW, SL, TH, GL, and HT.
The authors declare that they have no conflict of interest.
We thank Zheng Wei Zhang, Ky Su, Anita Eng, and Cassie Rauert for their support with the laboratory work. We thank Ewa Dabek and Jean-Pierre Charland for their support in obtaining the NAPS particulate matter samples.
This work was funded by Environment and Climate Change Canada and supports the Air Pollution and Chemicals Management programs. The work was also supported by the Max Planck Society.
This paper was edited by Pierre Herckes and reviewed by three anonymous referees.
Ayres, J. G., Borm, P., Cassee, F. R., Castranova, V., Donaldson, K., Ghio, A., Harrison, R. M., Hider, R., Kelly, F., Kooter, I. M., Marano, F., Maynard, R. L., Mudway, I., Nel, A., Sioutas, C., Smith, S., Baeza-Squiban, A., Cho, A., Duggan, S., and Froines, J.: Evaluating the toxicity of airborne particulate matter and nanoparticles by measuring oxidative stress potential – a workshop report and consensus statement, Inhal. Toxicol., 20, 75–99, https://doi.org/10.1080/08958370701665517, 2008.
Bates, J. T., Fang, T., Verma, V., Zeng, L., Weber, R. J., Tolbert, P. E., Abrams, J. Y., Sarnat, S. E., Klein, M., Mulholland, J. A., and Russell, A. G.: Review of acellular assays of ambient particulate matter oxidative potential: methods and relationships with composition, sources, and health effects, Environ. Sci. Technol., 53, 4003–4019, https://doi.org/10.1021/acs.est.8b03430, 2019.
Boisa, N., Elom, N., Dean, J. R., Deary, M. E., Bird, G., and Entwistle, J. A.: Development and application of an inhalation bioaccessibility method (IBM) for lead in the PM10 size fraction of soil, Environ. Int., 70, 132–142, https://doi.org/10.1016/j.envint.2014.05.021, 2014.
Borm, P. J. A., Kelly, F., Kunzli, N., Schins, R. P. F., and Donaldson, K.: Oxidant generation by particulate matter: from biologically effective dose to a promising, novel metric, Occup. Environ. Med., 64, 73–74, https://doi.org/10.1136/oem.2006.029090, 2007.
Bredberg, A., Gobom, J., Almstrand, A.-C., Larsson, P., Blennow, K., Olin, A.-C., and Mirgorodskaya, E.: Exhaled endogenous particles contain lung proteins, Clin. Chem., 58, 431–440, https://doi.org/10.1373/clinchem.2011.169235, 2012.
Burnett, R., Chen, H., Szyszkowicz, M., Fann, N., Hubbell, B., Pope, C. A., Apte, J. S., Brauer, M., Cohen, A., Weichenthal, S., Coggins, J., Di, Q., Brunekreef, B., Frostad, J., Lim, S. S., Kan, H., Walker, K. D., Thurston, G. D., Hayes, R. B., Lim, C. C., Turner, M. C., Jerrett, M., Krewski, D., Gapstur, S. M., Diver, W. R., Ostro, B., Goldberg, D., Crouse, D. L., Martin, R. V., Peters, P., Pinault, L., Tjepkema, M., van Donkelaar, A., Villeneuve, P. J., Miller, A. B., Yin, P., Zhou, M., Wang, L., Janssen, N. A. H., Marra, M., Atkinson, R. W., Tsang, H., Quoc Thach, T., Cannon, J. B., Allen, R. T., Hart, J. E., Laden, F., Cesaroni, G., Forastiere, F., Weinmayr, G., Jaensch, A., Nagel, G., Concin, H., and Spadaro, J. V.: Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter, P. Natl. Acad. Sci. USA, 115, 9592–9597, https://doi.org/10.1073/pnas.1803222115, 2018.
Calas, A., Uzu, G., Martins, J. M. F., Voisin, Di., Spadini, L., Lacroix, T., and Jaffrezo, J.-L.: The importance of simulated lung fluid (SLF) extractions for a more relevant evaluation of the oxidative potential of particulate matter, Sci. Rep., 7, 11617, https://doi.org/10.1038/s41598-017-11979-3, 2017.
Calas, A., Uzu, G., Kelly, F. J., Houdier, S., Martins, J. M. F., Thomas, F., Molton, F., Charron, A., Dunster, C., Oliete, A., Jacob, V., Besombes, J.-L., Chevrier, F., and Jaffrezo, J.-L.: Comparison between five acellular oxidative potential measurement assays performed with detailed chemistry on PM10 samples from the city of Chamonix (France), Atmos. Chem. Phys., 18, 7863–7875, https://doi.org/10.5194/acp-18-7863-2018, 2018.
Charrier, J. G. and Anastasio, C.: On dithiothreitol (DTT) as a measure of oxidative potential for ambient particles: evidence for the importance of soluble transition metals, Atmos. Chem. Phys., 12, 9321–9333, https://doi.org/10.5194/acp-12-9321-2012, 2012.
Charrier, J. G., McFall, A. S., Richards-Henderson, N. K., and Anastasio, C.: Hydrogen peroxide formation in a surrogate lung fluid by transition metals and quinones present in particulate matter, Environ. Sci. Technol., 48, 7010–7017, https://doi.org/10.1021/es501011w, 2014.
Cohen, A. J., Brauer, M., Burnett, R., Anderson, H. R., Frostad, J., Estep, K., Balakrishnan, K., Brunekreef, B., Dandona, L., Dandona, R., Feigin, V., Freedman, G., Hubbell, B., Jobling, A., Kan, H., Knibbs, L., Liu, Y., Martin, R., Morawska, L., Pope, C. A., Shin, H., Straif, K., Shaddick, G., Thomas, M., van Dingenen, R., van Donkelaar, A., Vos, T., Murray, C. J. L., and Forouzanfar, M. H.: Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015, Lancet, 389, 1907–1918, https://doi.org/10.1016/S0140-6736(17)30505-6, 2017.
Crobeddu, B., Aragao-Santiago, L., Bui, L.-C., Boland, S., and Baeza Squiban, A.: Oxidative potential of particulate matter 2.5 as predictive indicator of cellular stress, Environ. Pollut., 230, 125–133, https://doi.org/10.1016/j.envpol.2017.06.051, 2017.
Cross, C. E., Van der Vliet, A., O'Neill, C. A., Louie, S., and Halliwell, B.: Oxidants, antioxidants, and respiratory tract lining fluids, Environ. Health Persp., 102, 185–191, 1994.
Dou, J., Lin, P., Kuang, B.-Y., and Yu, J. Z.: Reactive oxygen species production mediated by humic-like substances in atmospheric aerosols: enhancement effects by pyridine, imidazole, and their derivatives, Environ. Sci. Technol., 49, 6457–6465, https://doi.org/10.1021/es5059378, 2015.
Escobar, J., Sánchez-Illana, Á., Kuligowski, J., Torres-Cuevas, I., Solberg, R., Garberg, H. T., Huun, M. U., Saugstad, O. D., Vento, M., and Cháfer-Pericás, C.: Development of a reliable method based on ultra-performance liquid chromatography coupled to tandem mass spectrometry to measure thiol-associated oxidative stress in whole blood samples, J. Pharm. Biomed. Anal., 123, 104–112, https://doi.org/10.1016/j.jpba.2016.02.007, 2016.
Fang, T., Verma, V., Bates, J. T., Abrams, J., Klein, M., Strickland, M. J., Sarnat, S. E., Chang, H. H., Mulholland, J. A., Tolbert, P. E., Russell, A. G., and Weber, R. J.: Oxidative potential of ambient water-soluble PM2.5 in the southeastern United States: contrasts in sources and health associations between ascorbic acid (AA) and dithiothreitol (DTT) assays, Atmos. Chem. Phys., 16, 3865–3879, https://doi.org/10.5194/acp-16-3865-2016, 2016.
Fang, T., Lakey, P. S. J., Weber, R. J., and Shiraiwa, M.: Oxidative potential of particulate matter and generation of reactive oxygen species in epithelial lining fluid, Environ. Sci. Technol., 53, 12784–12792, https://doi.org/10.1021/acs.est.9b03823, 2019.
Flohé, L.: The fairytale of the GSSG/GSH redox potential, BBA-Gen. Subjects, 1830, 3139–3142, https://doi.org/10.1016/j.bbagen.2012.10.020, 2013.
Giustarini, D., Tsikas, D., Colombo, G., Milzani, A., Dalle-Donne, I., Fanti, P., and Rossi, R.: Pitfalls in the analysis of the physiological antioxidant glutathione (GSH) and its disulfide (GSSG) in biological samples: an elephant in the room, J. Chromatogr. B, 1019, 21–28, https://doi.org/10.1016/j.jchromb.2016.02.015, 2016.
Gonzalez, D. H., Cala, C. K., Peng, Q., and Paulson, S. E.: HULIS enhancement of hydroxyl radical formation from Fe(II): kinetics of fulvic acid–Fe(II) complexes in the presence of lung antioxidants, Environ. Sci. Technol., 51, 7676–7685, https://doi.org/10.1021/acs.est.7b01299, 2017.
Gregory, T. J., Longmore, W. J., Moxley, M. A., Whitsett, J. A., Reed, C. R., Fowler, A. A., Hudson, L. D., Maunder, R. J., Crim, C., and Hyers, T. M.: Surfactant chemical composition and biophysical activity in acute respiratory distress syndrome, J. Clin. Invest., 88, 1976–1981, https://doi.org/10.1172/JCI115523, 1991.
Griese, M.: Pulmonary surfactant in health and human lung diseases: state of the art, Eur. Respir. J., 13, 1455–1476, https://doi.org/10.1034/j.1399-3003.1999.13f36.x, 1999.
Iriyama, K., Yoshiura, M., Iwamoto, T., and Ozaki, Y.: Simultaneous determination of uric and ascorbic acids in human serum by reversed-phase high-performance liquid chromatography with electrochemical detection, Anal. Biochem., 141, 238–243, https://doi.org/10.1016/0003-2697(84)90451-2, 1984.
Iyer, S. S., Jones, D. P., Brigham, K. L., and Rojas, M.: Oxidation of plasma cysteine/cystine redox state in endotoxin-induced lung injury, Am. J. Resp. Cell Mol., 40, 90–98, https://doi.org/10.1165/rcmb.2007-0447OC, 2009.
Johansson, J., Curstedt, T., and Robertson, B.: The proteins of the surfactant system, Eur. Respir. J., 7, 372–391, https://doi.org/10.1183/09031936.94.07020372, 1994.
Jones, D. P., Carlson, J. L., Mody, V. C., Cai, J., Lynn, M. J., and Sternberg, P.: Redox state of glutathione in human plasma, Free Radical. Biol. Med., 28, 625–635, https://doi.org/10.1016/S0891-5849(99)00275-0, 2000.
Kelly, F. J.: Oxidative stress: its role in air pollution and adverse health effects, Occup. Environ. Med., 60, 612–616, https://doi.org/10.1136/oem.60.8.612, 2003.
Kelly, F. J. and Fussell, J. C.: Role of oxidative stress in cardiovascular disease outcomes following exposure to ambient air pollution, Free Radical. Biol. Med., 110, 345–367, https://doi.org/10.1016/j.freeradbiomed.2017.06.019, 2017.
Künzli, N., Mudway, I. S., Götschi, T., Shi, T., Kelly, F. J., Cook, S., Burney, P., Forsberg, B., Gauderman, J. W., Hazenkamp, M. E., Heinrich, J., Jarvis, D., Norbäck, D., Payo-Losa, F., Poli, A., Sunyer, J., and Borm, P. J. A.: Comparison of oxidative properties, light absorbance, and total and elemental mass concentration of ambient PM2.5 collected at 20 European sites, Environ. Health Persp., 114, 684–690, https://doi.org/10.1289/ehp.8584, 2006.
Lelieveld, J., Haines, A., and Pozzer, A.: Age-dependent health risk from ambient air pollution: a modelling and data analysis of childhood mortality in middle-income and low-income countries, Lancet Planet. Heal., 2, 292–300, https://doi.org/10.1016/S2542-5196(18)30147-5, 2018.
Lelieveld, J., Klingmüller, K., Pozzer, A., Pöschl, U., Fnais, M., Daiber, A., and Münzel, T.: Cardiovascular disease burden from ambient air pollution in Europe reassessed using novel hazard ratio functions, Eur. Heart J., 40, 1590–1596, https://doi.org/10.1093/eurheartj/ehz135, 2019a.
Lelieveld, J., Klingmüller, K., Pozzer, A., Burnett, R. T., Haines, A., and Ramanathan, V.: Effects of fossil fuel and total anthropogenic emission removal on public health and climate, P. Natl. Acad. Sci. USA, 116, 7192–7197, https://doi.org/10.1073/pnas.1819989116, 2019b.
Li, N., Hao, M., Phalen, R. F., Hinds, W. C., and Nel, A. E.: Particulate air pollutants and asthma – a paradigm for the role of oxidative stress in PM-induced adverse health effects, Clin. Immunol., 109, 250–265, https://doi.org/10.1016/j.clim.2003.08.006, 2003.
Lodovici, M. and Bigagli, E.: Oxidative stress and air pollution exposure, J. Toxicol., 2011, 1–9, https://doi.org/10.1155/2011/487074, 2011.
Moore, T., Le, A., Niemi, A.-K., Kwan, T., Cusmano-Ozog, K., Enns, G. M., and Cowan, T. M.: A new LC–MS/MS method for the clinical determination of reduced and oxidized glutathione from whole blood, J. Chromatogr. B, 929, 51–55, https://doi.org/10.1016/j.jchromb.2013.04.004, 2013.
Mudway, I. S. and Kelly, F. J.: Modelling the interactions of ozone with pulmonary epithelial lining fluid antioxidants, Toxicol. Appl. Pharm., 148, 91–100, https://doi.org/10.1006/taap.1997.8318, 1998.
Paula, A. S., Matos, J. T. V, Duarte, R. M. B. O., and Duarte, A. C.: Two chemically distinct light-absorbing pools of urban organic aerosols: a comprehensive multidimensional analysis of trends, Chemosphere, 145, 215–223, https://doi.org/10.1016/j.chemosphere.2015.11.093, 2016.
Pison, U., Gono, E., Joka, T., Obertacke, U., and Obladen, M.: High-performance liquid chromatography of adult human bronchoalveolar lavage: assay for phospholipid lung profile, J. Chromatogr. B, 377, 79–89, https://doi.org/10.1016/S0378-4347(00)80763-X, 1986.
Pöschl, U. and Shiraiwa, M.: Multiphase chemistry at the atmosphere–biosphere interface influencing climate and public health in the Anthropocene, Chem. Rev., 115, 4440–4475, https://doi.org/10.1021/cr500487s, 2015.
Rossi, R., Milzani, A., Dalle-Donne, I., Giustarini, D., Lusini, L., Colombo, R., and Di Simplicio, P.: Blood glutathione disulfide: in vivo factor or in vitro artifact?, Clin. Chem., 48, 742–753, 2002.
Schafer, F. Q. and Buettner, G. R.: Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple, Free Radical. Bio. Med., 30, 1191–1212, https://doi.org/10.1016/S0891-5849(01)00480-4, 2001.
Song, Y. and Buettner, G. R.: Thermodynamic and kinetic considerations for the reaction of semiquinone radicals to form superoxide and hydrogen peroxide, Free Radical. Bio. Med., 49, 919–962, https://doi.org/10.1016/j.freeradbiomed.2010.05.009, 2010.
Stopford, W., Turner, J., Cappellini, D., and Brock, T.: Bioaccessibility testing of cobalt compounds, J. Environ. Monit., 5, 675–680, https://doi.org/10.1039/b302257a, 2003.
Tong, H., Arangio, A. M., Lakey, P. S. J., Berkemeier, T., Liu, F., Kampf, C. J., Brune, W. H., Pöschl, U., and Shiraiwa, M.: Hydroxyl radicals from secondary organic aerosol decomposition in water, Atmos. Chem. Phys., 16, 1761–1771, https://doi.org/10.5194/acp-16-1761-2016, 2016.
Tong, H., Lakey, P. S. J., Arangio, A. M., Socorro, J., Kampf, C. J., Berkemeier, T., Brune, W. H., Pöschl, U., and Shiraiwa, M.: Reactive oxygen species formed in aqueous mixtures of secondary organic aerosols and mineral dust influencing cloud chemistry and public health in the Anthropocene, Faraday Discuss., 200, 251–270, https://doi.org/10.1039/C7FD00023E, 2017.
Tong, H., Lakey, P. S. J., Arangio, A. M., Socorro, J., Shen, F., Lucas, K., Brune, W. H., Pöschl, U., and Shiraiwa, M.: Reactive oxygen species formed by secondary organic aerosols in water and surrogate lung fluid, Environ. Sci. Technol., 52, 11642–11651, https://doi.org/10.1021/acs.est.8b03695, 2018.
Tong, H., Zhang, Y., Filippi, A., Wang, T., Li, C., Liu, F., Leppla, D., Kourtchev, I., Wang, K., Keskinen, H.-M., Levula, J. T., Arangio, A. M., Shen, F., Ditas, F., Martin, S. T., Artaxo, P., Godoi, R. H. M., Yamamoto, C. I., de Souza, R. A. F., Huang, R.-J., Berkemeier, T., Wang, Y., Su, H., Cheng, Y., Pope, F. D., Fu, P., Yao, M., Pöhlker, C., Petäjä, T., Kulmala, M., Andreae, M. O., Shiraiwa, M., Pöschl, U., Hoffmann, T., and Kalberer, M.: Radical formation by fine particulate matter associated with highly oxygenated molecules, Environ. Sci. Technol., 53, 12506–12518, https://doi.org/10.1021/acs.est.9b05149, 2019.
Tuet, W. Y., Liu, F., de Oliveira Alves, N., Fok, S., Artaxo, P., Vasconcellos, P., Champion, J. A., and Ng, N. L.: Chemical oxidative potential and cellular oxidative stress from open biomass burning aerosol, Environ. Sci. Tech. Let., 6, 126–132, https://doi.org/10.1021/acs.estlett.9b00060, 2019.
Verma, V., Ning, Z., Cho, A. K., Schauer, J. J., Shafer, M. M., and Sioutas, C.: Redox activity of urban quasi-ultrafine particles from primary and secondary sources, Atmos. Environ., 43, 6360–6368, https://doi.org/10.1016/j.atmosenv.2009.09.019, 2009.
Verma, V., Fang, T., Guo, H., King, L., Bates, J. T., Peltier, R. E., Edgerton, E., Russell, A. G., and Weber, R. J.: Reactive oxygen species associated with water-soluble PM2.5 in the southeastern United States: spatiotemporal trends and source apportionment, Atmos. Chem. Phys., 14, 12915–12930, https://doi.org/10.5194/acp-14-12915-2014, 2014.
Verma, V., Wang, Y., El-Afifi, R., Fang, T., Rowland, J., Russell, A. G., and Weber, R. J.: Fractionating ambient humic-like substances (HULIS) for their reactive oxygen species activity – assessing the importance of quinones and atmospheric aging, Atmos. Environ., 120, 351–359, https://doi.org/10.1016/j.atmosenv.2015.09.010, 2015.
Weichenthal, S., Shekarrizfard, M., Traub, A., Kulka, R., Al-Rijleh, K., Anowar, S., Evans, G., and Hatzopoulou, M.: Within-city spatial variations in multiple measures of PM2.5 oxidative potential in Toronto, Canada, Environ. Sci. Technol., 53, 2799–2810, https://doi.org/10.1021/acs.est.8b05543, 2019.
Weichenthal, S. A., Godri Pollitt, K., and Villeneuve, P. J.: PM2.5, oxidant defence and cardiorespiratory health: a review, Environ. Health, 12, 40, https://doi.org/10.1186/1476-069X-12-40, 2013.
WHO: Ambient air pollution: a global assessment of exposure and burden of disease, World Health Organization, 2016.
Xiong, Q., Yu, H., Wang, R., Wei, J., and Verma, V.: Rethinking dithiothreitol-based particulate matter oxidative potential: measuring dithiothreitol consumption versus reactive oxygen species generation, Environ. Sci. Technol., 51, 6507–6514, https://doi.org/10.1021/acs.est.7b01272, 2017.
Zielinski, H., Mudway, I. S., Bérubé, K. A., Murphy, S., Richards, R.. and Kelly, F. J.: Modeling the interactions of particulates with epithelial lining fluid antioxidants, Am. J. Physiol.-Lung C., 277, 719–726, https://doi.org/10.1152/ajplung.1999.277.4.L719, 1999.