the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Evaluation of a field-deployable Nafion™-based air-drying system for collecting whole air samples and its application to stable isotope measurements of CO2
Hubertus A. Scheeren
Henk G. Jansen
Bert A. M. Kers
John B. Miller
Andrew M. Crotwell
Sylvia E. Michel
Luciana V. Gatti
Lucas G. Domingues
Caio S. C. Correia
Raiane A. L. Neves
Harro A. J. Meijer
Wouter Peters
Atmospheric flask samples are either collected at atmospheric pressure by opening a valve of a pre-evacuated flask or pressurized with the help of a pump to a few bar above ambient pressure. Under humid conditions, there is a risk that water vapor in the sample leads to condensation on the walls of the flask, notably at higher than ambient sampling pressures. Liquid water in sample flasks is known to affect the CO2 mixing ratios and also alters the isotopic composition of oxygen (17O and 18O) in CO2 via isotopic equilibration. Hence, for accurate determination of CO2 mole fractions and its stable isotopic composition, it is vital to dry the air samples to a sufficiently low dew point before they are pressurized in flasks to avoid condensation. Moreover, the drying system itself should not influence the mixing ratio and the isotopic composition of CO2 or that of the other constituents under study. For the Airborne Stable Isotopes of Carbon from the Amazon (ASICA) project focusing on accurate measurements of CO2 and its singly substituted stable isotopologues over the Amazon, an air-drying system capable of removing water vapor from air sampled at a dew point lower than −2 ∘C, flow rates up to 12 L min−1 and without the need for electrical power was needed. Since to date no commercial air-drying device that meets these requirements has been available, we designed and built our own consumable-free, power-free and portable drying system based on multitube Nafion™ gas sample driers (Perma Pure, Lakewood, USA). The required dry purge air is provided by feeding the exhaust flow of the flask sampling system through a dry molecular sieve (type 3A) cartridge. In this study we describe the systematic evaluation of our Nafion™-based air sample dryer with emphasis on its performance concerning the measurements of atmospheric CO2 mole fractions and the three singly substituted isotopologues of CO2 (16O13C16O, 16O12C17O and 16O12C18O), as well as the trace gas species CH4, CO, N2O and SF6. Experimental results simulating extreme tropical conditions (saturated air at 33 ∘C) indicated that the response of the air dryer is almost instantaneous and that approximately 85 L of air, containing up to 4 % water vapor, can be processed staying below a −2 ∘C dew point temperature (at 275 kPa). We estimated that at least eight flasks can be sampled (at an overpressure of 275 kPa) with a water vapor content below −2 ∘C dew point temperature during a typical flight sampling up to 5 km altitude over the Amazon, whereas the remaining samples would stay well below 5 ∘C dew point temperature (at 275 kPa). The performance of the air dryer on measurements of CO2, CH4, CO, N2O, and SF6 and the CO2 isotopologues 16O13C16O and 16O12C18O was tested in the laboratory simulating real sampling conditions by compressing humidified air from a calibrated cylinder, after being dried by the air dryer, into sample flasks. We found that the mole fraction and the isotopic composition difference between the different test conditions (including the dryer) and the base condition (dry air, without dryer) remained well within or very close to, in the case of N2O, the World Meteorological Organization recommended compatibility goals for independent measurement programs, proving that the test condition induced no significant bias on the sample measurements.
- Article
(4844 KB) - Full-text XML
- BibTeX
- EndNote
Carbon dioxide (CO2) is one of the most important and extensively monitored greenhouse gases in the atmosphere. Atmospheric CO2 dry air mole fraction measurements provide information that helps understand the continuously increasing mole fractions in the atmosphere due to the combination of human activities and exchange with the terrestrial and oceanic components of the global carbon cycle. Further, measurements of the isotopic composition of the atmospheric CO2 provide information about its sources and sinks. CO2 mole fraction can be continuously measured using instruments capable of performing continuous-flow measurements in whole air samples, e.g., using nondispersive infrared (NDIR)-based sensors (Stephens et al., 2011), using cavity ring-down spectrometers (Chen et al., 2010) or quasi-continuously by using gas chromatography (van der Laan et al., 2009). Alternatively, discrete air samples can be collected in flasks for later analysis in a laboratory. Flasks are typically filled with ambient air either by opening the valve of a pre-evacuated flask or by pressurizing a flask with the help of a pump. Under humid conditions, flask sampling requires drying of the sample air to prevent condensation inside the flask, which can affect the CO2 mole fractions as well as the oxygen stable isotope composition (Gemery et al., 1996; Trolier et al., 1996).
Since 2009, a substantial effort has been undertaken to establish a long-term atmospheric mole fraction CO2 record over the Amazon rain forest. Air samples are collected on board a small aircraft along a vertical profile from 4.4 km down to 300 m a.m.s.l. (above mean sea level) at a bimonthly (twice a month) rate at four different sites (Alta Floresta, ALF; Rio Branco, RBA; Santarém, SAN, and Tefé, TEF) over the Amazon forest. Additionally, samples are also collected once every month at Manaus (MAN) and over a big flooded area in a different ecosystem at Pantanal (PAN, Mato Grosso state). This unique CO2 program resulted in a number of new insights on net carbon exchange from this region (Alden et al., 2016; Gatti et al., 2014; van der Laan-Luijkx et al., 2015), and the measurements continue still. The project Airborne Stable Isotopes of Carbon from the Amazon (ASICA, http://www.asica.eu, last access: April 2019) aims to add a record of high-precision measurements of the singly substituted isotopologues of CO2 to determine δ13C, δ17O and δ18O of the atmospheric CO2. Whereas the former isotope ratio potentially constrains the water-use efficiency of CO2 exchange (Keeling et al., 2017; Peters et al., 2018), the latter two isotope ratios can be combined into so-called “excess 17O in CO2” (Δ17O), which has shown early potential to serve as a tracer of leaf–atmosphere exchange of CO2 (Hoag et al., 2005; Hofmann et al., 2017; Koren et al., 2019), with potential new insights on the gross carbon uptake through photosynthesis of this vast forest area. Removal of water vapor from the sampled air is of utmost importance, as oxygen isotopes can easily equilibrate between CO2 and liquid water, destroying the signature of Δ17O we are after in ASICA. To perform reliable measurements of CO2 and its isotopologues, an efficient inline dryer thus had to be included in our flask sampling system.
Here we describe the development and testing of a field-deployable, consumable-free, power-free and portable drying system based on multitube Nafion™ gas sample driers (Perma Pure, Lakewood, USA). Nafion™ is a copolymer of perfluoro-3,6-dioxa-4-methyl-7-octene-sulfonic acid and tetrafluoroethylene, which is known for its unique selectivity and high permeability for water (Perma-Pure-LLC, 2019). In the form of a membrane, this property enables it to transfers moisture from the sample gas stream to a counter-flowing dry purge gas stream. The design of the Nafion™ air dryer, hereon referred to as NAD, consists of two Perma Pure PD-Series™ gas dryers containing 200 Nafion™ tubes each in a stainless steel tube shell (PD-200T-24MSS) placed in series (as shown in Fig. 1). A counter flow of dry purge gas within the shell removes moisture from the sample air stream permeating through the tubing. The dry purge air is provided by feeding the exhaust flow of the programmable flask package (PFP) through a 350 g dry molecular sieve (type 3A) cartridge, which effectively removes water to a dew point better than −5 ∘C at STP conditions. The NAD is designed to be part of the standard sampling system used in Brazil, as well as in the cooperative air sampling program in the United States, which consists of a programmable compressor package (PCP) and a programmable flask package (version 3) containing twelve 700 mL (Sweeney et al., 2015) flasks. Since February 2018, the first NAD used also in the tests described here has been in actual use at RBA, while two more will be deployed at ALF in the second half of 2019.
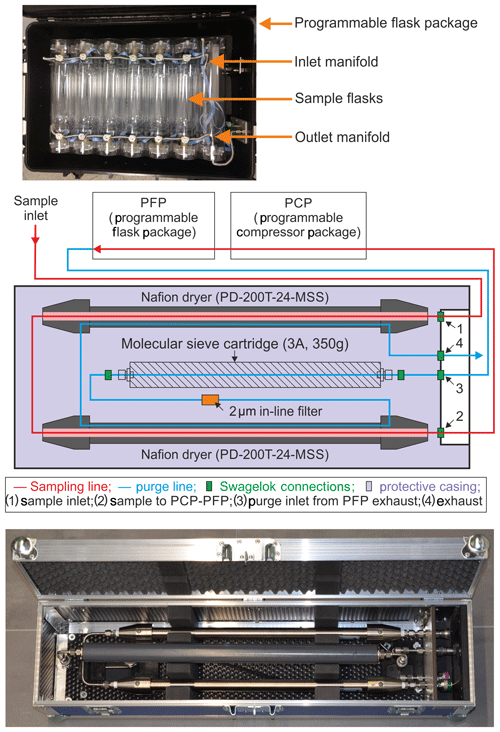
Figure 1Top: a programmable flask package showing the flasks and the inlet/outlet manifolds. Middle: a schematic showing the Nafion™ dryer system, which is housed inside a custom-built box, and the flow pathway therein. The Nafion™ dryer system is comprised of two Nafion™ dryers (Perma Pure PD-200T-24-MSS), a molecular sieve cartridge, a 2 µm in-line filter, and various Swagelok connectors and tubing. Ambient air first passes through the two conditioned Nafion™ dryers connected in series (red line), drying the incoming air. It then goes through the PCP, which pushes the sample through the PFP. The exhaust of the PFP is connected to the molecular sieve cartridge in the NAD, which removes any residual moisture in the air directed into the Nafion™ purge (blue line). The purge flow through the Nafion™ is in a direction opposite to the flow of the sample air, and the dry purge air ensures that the Nafion™ is able to dry at least 230 L of sample air that flows through the system during a typical flight sampling. Bottom: picture of the Nafion™ air dryer in a custom-built casing ( cm).
We present the results of extensive experiments testing the drying capacity, as well as the stability and potential interference, of the NAD on CO2 mole fractions and its singly substituted isotopologues and on other important atmospheric trace gases such as CH4, CO, SF6 and N2O. The drying capacity and endurance of the Nafion™ tubes in combination with the molecular sieve drier cartridge were examined under laboratory test conditions with sample air containing ∼4 % H2O at 33 ∘C, resembling extreme tropical conditions. Isotopic measurements of sample air fed through the NAD were performed using the dual-laser tunable infrared laser direct absorption spectrometer (TILDAS, Aerodyne Research, Inc.) at Groningen, specially designed for the measurements of all the three singly substituted CO2 isotopologues, 16O13C16O, 16O12C17O and 16O12C18O, in whole air samples (McManus et al., 2015; Sakai et al., 2017). Finally, we present the results of a series of performance tests of the NAD in combination with a PCP and PFP sampling system simulating field conditions.
2.1 Analytical techniques
All continuous observations of CO2 mole fractions in air flowing through the NAD at the CIO (Centre for Isotope Research, University of Groningen) and INSTAAR (Institute of Arctic and Alpine Research, University of Colorado) were conducted using a cavity ring-down spectrometer for CO2, CH4 and H2O (CRDS, Picarro, Inc., CA, model G2301) (Crosson, 2008). The overall measurement precision of the CRDS systems used was typically <0.03 µmol mol−1 (ppm) for CO2; <0.2 nmol mol−1 (ppb) for CH4, based on our long-term measurements of target cylinders; and <30 ppm for H2O, based on manufacturers specifications.
At the CIO we employed a dual-laser tunable infrared laser direct absorption spectrometer (TILDAS, Aerodyne Research, Inc.) (McManus et al., 2015; Sakai et al., 2017), which we refer to as the TILDAS-SICAS (SICAS for stable isotopes of CO2 measurements in atmospheric samples), for the measurements of 16O13C16O, 16O12C17O and 16O12C18O in whole air samples. More details about the TILDAS-SICAS setup and performance will be described elsewhere in a forthcoming paper. The isotopic composition of the CO2 in the sample gas with respect to the reference gas is determined using the following equations for 16O13C16O, 16O12C17O and 16O12C18O:
The flask samples from the NAD testing replicating field condition experiments at INSTAAR were analyzed at NOAA-ESRL (National Oceanic and Atmospheric Administration Earth System Research Laboratory) for CO2, CH4, CO and SF6 on the Measurement of Atmospheric Gases that Influence Climate Change (MAGICC) system. CO2 is measured on a LI-COR® nondispersive infrared analyzer with a precision of ±0.03 ppm (Conway et al., 1994), and CH4 and CO are measured by gas chromatography followed by flame-ionization detection for CH4 with a precision of ±1.2 ppb (Dlugokencky et al., 1994). CO is measured by vacuum ultraviolet resonance fluorescence spectroscopy with a precision of ±0.3 ppb (Gerbig et al., 1999). N2O and SF6 are measured by gas chromatography followed by electron capture detection with a precision of 0.3 ppb and 0.04 ppt, respectively. All gases are measured relative to suites of working standards that are directly linked to the World Meteorological Organization (WMO) primary standard scales. A copy of the MAGICC system as well as an Aerodyne TILDAS is present at the Greenhouse Gas Laboratory (LaGEE) at INPE, São José dos Campos, Brazil (Gatti et al., 2014), for the analysis of the flask samples collected over the Brazilian Amazon as part of the ASICA project.
For the analysis of the isotopic composition of CO2 (δ13C and δ18O) in the PFP samples from the NAD testing experiments at INSTAAR, CO2 was extracted from ∼450 mL of sample air. This extracted CO2 was then analyzed on a dual-inlet isotope ratio mass spectrometer (DI-IRMS) (Isoprime, Elementar, Middlewich, UK) at INSTAAR and compared against working references linked to the INSTAAR realization of the VPDB-CO2 scale. The precision of δ13C and δ18O values of CO2 on the DI-IRMS was ±0.02 ‰ and ±0.04 ‰, respectively (Vaughn et al., 2004).
2.2 Design and operation of the Nafion™ air-drying system
The NAD was developed and tested at the Centre for Isotope Research (CIO), University of Groningen, the Netherlands. Additional experiments were done at the National Oceanic and Atmospheric Administration Earth System Research Laboratory (NOAA-ESRL) and the Institute of Arctic and Alpine Research (INSTAAR), University of Colorado, both in Boulder, Colorado, USA. The NAD has been designed to operate together with the NOAA-ESRL air sampling system consisting of a programmable compressor package (PCP) for flushing and pressurizing the samples (typically to 275 kPa) and a programmable flask package (PFP) containing twelve 700 mL borosilicate glass flasks to contain the samples. The top panel of Fig. 1 shows a picture of a PFP with the inlet/outlet manifolds and the flasks. All the sample flasks are connected through the manifold, and each flask has its own inlet and outlet valve, controlled by a computer. There are several version of the PFP/PCP available and in use at INPE, some with an outlet manifold (version 2, similar to the one shown in Fig. 1) and some without an outlet manifold (version 3) that vented the outlet flow into the PFP box itself. To make the version 3 PFPs compatible with the NAD, an outlet manifold was added to this system, which is the one shown in Fig. 1.
The NAD contains two Perma Pure PD-Series™ Nafion™ dryers (PD-200T-24-MSS), a molecular sieve cartridge (type 3A, ∼2 mm beads, 350 g, Sigma Aldrich), a 2 µm in-line filter (Swagelok, SS-4FW-2), stainless steel tubing and various Swagelok connectors. The middle panel of Fig. 1 shows a schematic of all the components inside the dryer along with the flow path. The bottom panel shows a picture of the complete Nafion™ air-drying system. Sample air enters the system through a tubing connected on quick-connect connector 1 (SS-QC4-S-200K3) and makes its way through the two Nafion™ dryers connected in series, into tubing connecting quick-connect connector 2 (SS-QC4-B1-400) to the PCP. The PCP then pushes the air through the PFP. The exhaust of the PFP is then directed back to the NAD through a tube to quick-connect connector 3 (SS-QC4-S-200K7) where the molecular sieve cartridge is connected. The air then travels through the molecular sieve cartridge in order to dry the exiting air stream going into the purge inlet of the Nafion™ dryers in a direction opposite to that of the sample flow, and finally it exits the system through the exhaust line connected on quick-connect connector 4 (SS-QC4-D1-400). During an actual flask sampling, the exhaust of the flask, which is used as a purge flow, is stopped because the outlet of the flask is closed to pressurize the flask. We note that the required capture of the outflow on quick-connect connector 3 could be easily accommodated by retrofitting the back manifold on the PFP (version 3), while the older PFPs (version 2) could not readily be used, partially because of their much higher flow rates of up to 40 L min−1. The NAD system is housed inside a rugged case (custom-built by Dutycases, Drachten, the Netherlands), as shown in the bottom panel of Fig. 1.
The Nafion™ dryers were conditioned before every use by passing dry air (from a compressor with a water content typically <0.02 % H2O by volume) through both the sampling line and the purge line for a period of about 12 h (typically overnight) at 2 slpm (standard liters per minute). A hygrometer (Rotronic HygroPalm HP22-A) and a CRDS (Picarro, model G2301) were used to monitor the relative humidity of air exiting the Nafion™ dryers during the conditioning procedure. The drying process was completed when the relative humidity of the outgoing air was <0.02 % H2O by volume as measured on the CRDS and reading 0 % RH on the hygrometer. The time required for drying the Nafion™ dryers and the ultimate dryness varied depending on the humidity conditions they were previously exposed to and the moisture content of the drying air used for conditioning the NAD. For atmospheric conditions of ∼20 ∘C and ∼2 % H2O, we found that a 10 h flushing period was more than sufficient to dry the NAD to an H2O level <0.02 % in the outgoing air. Similarly, the molecular sieve granules were also regenerated after every use by drying them overnight in an open beaker at 100 ∘C. This amount of the molecular sieve (∼350 g) and the method of regeneration was more than sufficient for experiments that would mimic the sample collection procedure over the Brazilian Amazon; i.e., the molecular sieve was sufficient to remove equivalent quantities of water amounting to ∼10 g of H2O. Since the process of adsorption of water on the molecular sieve is exothermic, it was conveniently followed using an infrared camera (FLIR i7) to determine an approximate minimum quantity of the adsorbent material required (∼175 g).
Following the drying step, the NAD system was filled with dry air at ambient pressure. Since all the Swagelok® quick-connect connections used in this system are of the normally closed type, the system can thus be stored for several weeks without having to recondition them before use. We successfully tested storage times up to 1 month in the laboratory. This is especially useful for the sampling strategy employed for the ASICA samples, where the conditioning of the NAD is performed in the laboratory (CCST/INPE, São José dos Campos, Sao Paulo) and is then stored for a few days before it is shipped to a site for sample collection. The NAD and the PFP are sent to the sample site together for sample collection, and then both are returned to the laboratory for analysis and conditioning. Note that the PCPs remain at the airport, and residual water vapor in this component (as well as in the tubing of the aircraft) needs to be removed during the system start-up tests on the ground, performed prior to each flight after a preconditioned NAD is attached. During this preflight test, ∼5 L of outside air is flushed through the full inlet system (all lines + PFP manifold + PCP + NAD) while the humid outside air is predried with a small handheld molecular sieve 3A cartridge attached to the wing inlet, to limit loss of NAD capacity before take-off and actual flask sampling.
3.1 Development and design of the Nafion™ air dryer
When atmospheric samples are collected under humid conditions, there is a risk that the water vapor in the sample leads to condensation on the walls of the inlet tubing or flask especially at higher than ambient sampling pressures. This leads to an oxygen isotopic equilibration between CO2 and H2O, effectively setting the δ18O and δ17O in CO2 to that of the more abundant water vapor. To avoid condensation of water inside the PFP sampler, it is necessary to dry the samples to a sufficiently low dew point before storage. For the ASICA program air samples are collected over the Brazilian Amazon region where the air can be close to saturation (>90 % relative humidity) at a temperature up to 35 ∘C. These samples are susceptible to condensation when brought back to the laboratory with a typical indoor temperature of ∼20 ∘C.
For the ASICA project, typically 12 flasks are filled with dried air during each flight sampling. Flask filling is initiated by toggling a switch that initiates the pumps in the PCP and switches flask valves in the PFP. The inlet tubing, NAD system, PCP and PFP manifold are first flushed with 5 L of air, followed by opening of the valves and flushing of the flask with 10 L of air. A sample is collected by closing the downstream flask valve and pressurizing the flask to 275 kPa (absolute) before closing the upstream valve (corresponding to ∼1.9 L of air at STP). The total amount of air that is dried per sample, from flushing to sampling, is around ∼17 L, adding up to ∼204 L for one flight of 12 samples, excluding the preflight test which is done with predried air.
The first drying method we tested was an in-line magnesium perchlorate (Mg(ClO4)2) packed cartridge to dry humidified air, which at first seemed to be an easier alternative to the NAD to remove water vapor from the sample stream on board a small aircraft. Magnesium perchlorate is a desiccant and is capable of drying air samples without affecting its composition (notably of CO2 and its isotopic composition), as observed during our initial tests. It can be regenerated by heating at 220 ∘C () under vacuum. Theoretically, to dry around 200 L of humidified air containing 4 % water vapor, a minimum of 12.4 g anhydrous Mg(ClO4)2 would be sufficient. However, the use of Mg(ClO4)2 includes a number of disadvantages that led us to look for an alternative better suited for the ASICA program. The first disadvantage of using magnesium perchlorate concerns safety and health hazards inherent in perchlorates. Perchlorates are stable at normal temperatures, but when they are exposed to high temperatures, e.g., in case of a fire, they accelerate combustion. Secondly, in case of an accident, exposure to perchlorates can cause serious skin, eye and respiratory irritations. Hence, usage on board an aircraft is mostly prohibited or at least restricted to an amount too small for our purpose. Another drawback of using Mg(ClO4)2 is that it tends to change into a thick slurry when retaining significant amounts of water, which eventually restricts the sample flow and in the worst case could block the flow completely. Finally, it is difficult to regenerate it to its original grain size after usage, so typically each flight would require a fresh batch.
As a result, we decided to move away from Mg(ClO4)2 to dry our sample air and started experimenting with multitube Nafion™ gas sample driers from Perma Pure. Due to the relatively high flow rate of the PCP–PFP sampling system of up to 15 L min−1, we chose to use the 24′′ Perma Pure PD-Series™ gas dryers containing 200 Nafion™ tubes each, in a stainless steel tube shell designed for high flows up 40 L min−1. To dry a sample flow, a counter-flowing dry purge air is needed. According to manufacturer's recommendations, optimal result with one PD-Series™ tube would be achieved when purge air of −40 ∘C dew point can be offered at a flow rate of 2 to 3 times the sample flow at a pressure equal to or lower than the sample flow (Perma-Pure-LLC, 2019). However, this would require an additional dry air tank containing at least 600 L of compressed air on board each flight, which is undesirable from both a logistic and safety point of view. When no dry purge gas is available, one can choose to reuse the sample gas itself after it is partially dried passing through the Nafion™ tube (Welp et al., 2013) or, for a lower dew point, with an additional water trap such as a freeze dryer/molecular sieve to remove the remaining water before it is reused as purge gas (e.g., Neubert et al., 2004; Stephens et al., 2011). Although an excellent desiccant by itself, a molecular sieve (type 3A) cannot be used to directly dry sample air as it tends to alter the composition of air. Hence, we chose to use a molecular sieve (type 3A) as a drying agent in the purge flow line because it is additionally nontoxic, economical and reusable.
Besides the number of Nafion™ tubes inside the shell and the dew point of the purge air, the performance of the Nafion™ tube is dependent on the dryer length and both the sample and purge flow rates. In our setup, the sample flow rates are typically ∼12 L min−1 (sample flow = purge flow). According to the PD-200T-24MSS specifications of the manufacturer (Perma Pure, USA), a sample stream of 12 L min−1 with a dew point of 20 ∘C would require a dry purge flow at 2 times the sample flow rate to get to a dew point of −12 ∘C. For the samples collected over the Amazon basin, the dew point could be as high as 30 ∘C, and the maximum possible flow rate we can offer to the purge line is equal to the sample flow rate. Additionally, since the samples are compressed to 275 kPa, the water vapor content in the samples has to be even lower than at STP conditions as discussed earlier. As a single 24′′ Nafion™ dryer would therefore not be sufficient to achieve the required water vapor content in all the 12 sample flasks, we decided to instead use two 24′′ Nafion™ dryers in series to increase the effective interaction length to 48′′, thus ensuring acceptable levels of water vapor content in the sampling flasks. A previous study (Gemery et al., 1996) recommended that, to obtain reliable measurements of oxygen isotopes in CO2, one needs to dry flask samples to better than 2 ∘C dew point for flasks filled to atmospheric pressure (RH≈30 % at 20 ∘C). The authors noted that the largest deviations in δ18O were observed only when the relative humidity in the flasks was above 100 % at conditions where water would condense on the wall of the flask. No significant deviations were observed for flasks containing lower than 60 % relative humidity, whereas small deviations were observed between 60 % and 100 % RH. The deviations observed above 60 % relative humidity gradually increased and were >0.5 ‰ above 80 % relative humidity.
We used this study to set our boundary conditions in order to achieve reliable δ18O measurements. Thus, a flask filled at 275 kPa with 100 % RH (at 20 ∘C) corresponds to a dew point temperature of ∼5 ∘C and that with 60 % RH (at 20 ∘C) to a dew point temperature of ∘C. We have monitored the temperature experienced inside the PFP since the time it is sent to the sample collection site to the time it is brought back to the lab, for a period of approximately 2 years, with the help of a portable temperature sensor (Omega Engineering, OM-EL-USB-1). These temperature profile data indicate that the minimum temperature the PFP experiences in the Amazonian sample collection sites is about 11 ∘C. As discussed and shown later in this paper, most samples collected in the Amazonia have a water vapor content lower than −2 ∘C dew point (∼60 % RH), and none would exceed the 5 ∘C dew point (∼100 % RH) limit at 275 kPa flask pressure. In summary, for an optimal performance under tropical saturated conditions, we decided to use the PD-200T with a total length of 48′′ (∼140 cm). For practical reasons we chose to use two of the 24′′ (∼70 cm) long PD-200T-24MSS, which can be placed in series (in a parallel configuration) to save space.
The amount of the molecular sieve needed to sufficiently dry the purge gas was initially set to ∼150 g. From our experiments testing the drying capacity of the double Nafion™ tube, we found that a minimum of 175 g of the dry molecular sieve was needed for drying a minimum of 200 L of air at moderate conditions of ∼22 ∘C and 70 % RH down to <1 % RH. For a safe margin with respect to the humid tropical conditions we meet over the Amazon region, we decided to double the amount of the molecular sieve to 350 g. Our work on testing the drying capacity of the NAD is further elaborated in the next section.
3.2 Drying capacity of the NAD
The drying capacity of Nafion™ is dependent on the dryness of the purge flow and the individual Nafion™ tubes. The hydrophilic properties of sulfonated tetrafluoroethylene polymer, from which Nafion™ is formed, absorb water molecules at the humid side and release them at the dry side, creating a permeation of water from the wet side to the dry side. When the Nafion™ material is not properly dried before usage, the material will remain saturated with water molecules for a longer period of time and will thus limit its total capability to dry the sample air stream. Hence, before each experiment or operation with the NAD, it was essential to dry the Nafion™ tubes by flushing them with dry compressed air at a rate of 2 L min−1 at STP for about 12 h. After this time, the outflow of the NAD would show no remaining water vapor; i.e., the outflow equals the inflow water vapor content, indicating the Nafion™ is dry.
Here we describe the results of a specific experiment where we mimicked the sample collection process during a flight under tropical conditions to demonstrate the usability and the performance of the NAD. This experiment also allowed us to determine the total amount of water the NAD was capable of removing in its current setup. The experiments were performed in a greenhouse test facility at the biology department of the University of Groningen, where the water content of the sampled air could be set to 3.7 %–3.9 % at ∼33 ∘C mean temperature. As described in Sect. 3.1, a sampling sequence using the PCP–PFP system consists of 5 L of air for flushing the PCP–PFP inlet and manifold, followed by flushing the sample flask with 10 L of air, and ending with pressurizing the sample flask up to 275 kPa before closing the upstream valve, corresponding to ∼1.9 L of air at STP in the flask. The complete filling of a flask was simulated by flushing the NAD periodically at 6 L min−1 with ambient air from the greenhouse for a duration of 3 min, depicted in Fig. 2. The bottom left axis in Fig. 2 shows the water vapor content, as measured by the Picarro CRDS; the top left axis shows the corresponding dew point temperature at 275 kPa; and the right axis shows the total volume of air processed through the NAD. The first 50 s correspond to the inlet and manifold flushing (blue points in the dew point data), the next 100 s refer to the flushing of the flask plus inlet and manifold (red points), and the filling resembled the last 20 s (green points). Although we tried to process approximately 17 L of humidified air in every step, similar to a real sampling scenario, there was a slight amount of excess air that was processed in every step (pink points). With a targeted maximum dew point of −2 ∘C, we found that the drying unit is capable of drying four samples, corresponding to approximately ∼85 L of air containing 3.7 %–3.9 % H2O. The drying capacity of the NAD is related to both the rate of saturation of the Nafion™ tubes and also the amount of the molecular sieve used. At high-humidity conditions both the Nafion™ tubes and the molecular sieve cartridge gets saturated very fast and ultimately affects the number of samples that can be processed. We note that this can be considered as a worst-case scenario relative to the average conditions met at the ASICA sampling program where typically 6 out of 12 flasks are filled below 2 km altitude in the tropical boundary layer, and the remaining flasks are collected in the dryer middle troposphere up to about 5 km altitude.
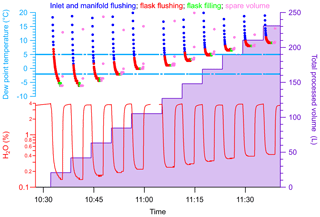
Figure 2A time series mimicking the sample collection process during a flight to demonstrate the usability and the response of the Nafion™ dryer under conditions similar to those of the tropics (sample air with 3.7 %–3.9 % water vapor content at ∼33 ∘C). The bottom panel (left) shows the water vapor content in the sampled air, the top panel (left) shows the corresponding dew point temperature (at 275 kPa) and the panel on the right shows the total processed volume. Periodically, humid air from the test facility was passed through the NAD, indicated by the dips in the water vapor content (%) of the sampled air, to simulate a complete filling step of a PFP flask. These steps are indicated in the dew point plot as follows: (1) flushing of the inlet and the manifold volume (blue points), (2) flushing of the inlet and manifold volume plus the flask (red points), and (3) filling of a flask (green points). A slight amount of excess air was processed in every passing through the Nafion™ dryer, indicated by the pink points. The targeted dew point temperature was −2 ∘C, and as can be seen, the drying unit is capable of drying approximately 85 L of air containing 3.7 %–3.9 % H2O.
From the data presented in Fig. 2, we estimated the total amount of accumulated water after which the NAD is not capable of delivering samples dryer than the −2 ∘C dew point (at 275 kPa) is 2.4–2.5 g. In Fig. 3 we present a typical sampling profile over the Amazon as a function of altitude and the estimated amount of water removed by the NAD system. The bottom axis shows the amount of water removed (g) by the dryer, and the top axis shows the water vapor content in the sample at a given altitude (blue solid line). The water vapor content at each 1 km altitude bin was taken from sounding data (Soundings-Data, 2019) collected during the wet seasons over Rio Branco and Santarém and represented the maximum values observed in that altitude bin. Of course, the water vapor content gradually decreases as a function of altitude, and the calculated amount of water removed, as shown in Fig. 3, is thus an overestimate.
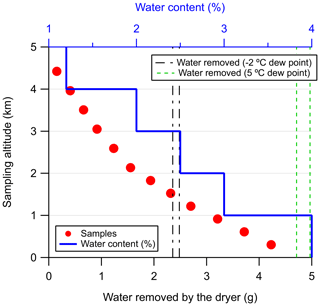
Figure 3A simplified estimation of the total amount of water that the Nafion™ dryer has to remove during a typical flight over the Amazon. Twelve samples are collected gradually from a height of about 4.4 km down to about 300 m (a.m.s.l.). The water vapor content in the air at a given altitude is shown with the solid blue line. This value has been assumed constant over every 1 km altitude bin and was the maximum value observed in the sounding data, collected during the wet season over Rio Branco and Santarém, over the altitude bin. The black and green dashed lines indicate the lower and upper boundaries for the number of samples that can be collected with a dew point lower than −2 ∘C (at 275 kPa) and 5 ∘C (at 275 kPa), respectively. These lower and upper boundaries were estimated from the experiment shown in Fig. 2.
Sampling is gradually carried out from an altitude of about 4.4 km down to about 300 m, and 12 samples are typically collected during such a flight and are shown with the red points. The two black and green dashed lines indicate the lower and upper boundaries, determined from the data presented in Fig. 2, for the number of samples that can be collected with a dew point lower than −2 ∘C (60 % RH) and 5 ∘C (100 % RH), respectively. This estimation shows that for a typical flight, the accumulated water in the NAD stays well below the worst-case scenario approximated by the greenhouse experiments where only four samples could be collected before reaching the −2 ∘C dew point threshold. Figure 3 further indicates that at least eight samples can be collected under typical flight conditions with dew points below −2 ∘C and the last four with dew points well below 5 ∘C.
3.3 Effect of the NAD on CO2 isotopic composition
To determine the effect of the Nafion™ dryer on the isotopic composition of CO2 in an air sample, a zero-enrichment (ZE) experiment was performed, similar to the ones performed with a dual-inlet IRMS (Wright et al., 1983). For the ZE experiments, air from a compressed air tank was treated both as a reference gas (unprocessed: dry) and a sample gas (processed: humidified). Thus, in a ZE experiment, if the isotopic composition of the reference gas is identical to that of the isotopic composition of the sample gas, the resultant difference is zero (17δ, 18δ, and 13δ=0 ‰; see Eqs. 1–3 Sect. 2.1), indicating no effect.
To determine the isotopic composition of CO2 in air and the influence of the Nafion™ dryer on the stable isotopes of CO2, we used our TILDAS-SICAS, which was designed to detect the CO2 isotopologues 16O12C16O, 16O13C16O, 18O12C16O and 17O12C16O in whole air samples. All the measurements performed on the TILDAS-SICAS were static measurements; i.e., a specific volume of air is introduced into the optical cell and a measurement is then performed for a period of 60 s, after which the optical cell is evacuated and the next sample is introduced. This measurement scheme allowed a semicontinuous measurement mode for all the stable isotopes of CO2 to investigate the effect of the NAD on the isotopic composition of the downstream CO2 as a function of time. For the reference measurements, the gas was directly fed into the TILDAS-SICAS for the measurements of the mole fractions of all the stable isotopologues of CO2. For the sample measurements, air from the same cylinder was either sent into the TILDAS-SICAS through the NAD (dry mode) or it was first humidified (∼2 %) and then dried using the Nafion™ dryer (wet mode), before sending the air stream into the TILDAS-SICAS for the measurements of the mole fractions of all the previously mentioned isotopologues of CO2. The sample air stream was humidified at room temperature, by passing the dry cylinder air through a bubbler containing demineralized water. The nozzle of the bubbler was constructed using a sintered glass filter to maximize the water content in the downstream sample air, reaching a maximum of ∼2 % at room temperature. Although the humidity level achieved in these experiments was less than the maximum one would encounter in the Brazilian Amazon (0–3 km), it clearly demonstrates the lack of isotopic exchange caused by the interaction of CO2 with the oxygen-rich Nafion™ surface in the dry mode and in a relatively less severe wet mode. Indeed, further experiments with sample air saturated with water vapor, up to ∼4 %, would be needed to confirm a complete lack of isotopic exchange even at high-humidity conditions.
One such experiment is illustrated in Fig. 4, where the CO2 mole fraction is shown in the top panel, and the calculated δ17O, δ18O and δ13C are shown in the subsequent panels below the CO2 mole fraction panel. The calculated δ17O, δ18O, and δ13C values for the sample measurements are determined with respect to the reference measurements performed before and after each sample measurement. This measurement strategy is similar to the ones used for IRMS-based measurements, which eliminates systematic instrument drifts during a reference–sample–reference measurement set. The first part of the experiment (shown with a red background) was performed in dry mode, and the second part (shown with a blue background) was performed in wet mode. The section between the dry and the wet mode, shown with a white background, denotes a stabilization period during which the cylinder air was humidified and the wet air stream was passed through the NAD. The total volume of air processed in the dry mode and wet mode (including the stabilization period) was 162 and 174 L, respectively, at a flow rate of 4.5 L min−1. As can be seen from the data in Fig. 4, the isotopic composition of CO2 in the cylinder air is not altered by the NAD, both in dry and wet conditions. One sample measurement (measurement number 16) in the dry mode was affected by an unknown cause and was considered an outlier – thus not included in the analysis. The means of the ZE measurements (± the standard error of the mean), corresponding to the dry and wet mode, for the three singly substituted isotopologues of CO2 are also shown in Fig. 4. We note that the flow rate used during this experiment (4.5 L min−1) is significantly lower than the flow rates typically used during sample collection (∼12 L min−1). This clearly demonstrates that under laboratory test conditions the NAD has a negligible effect on the isotopic composition of CO2, even with significantly longer residence times in the Nafion™ tubes. It is thus expected that, at higher flow rates (12 L min−1), the reduced interaction time between the air stream and the NAD surface should have even lesser influence on the isotopic composition of CO2.
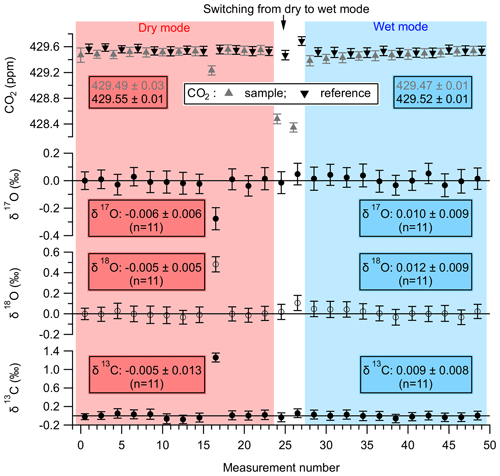
Figure 4A zero-enrichment experiment performed with the TILDAS-SICAS to test the effect of the NAD on the CO2 mole fraction and the stable isotopes of CO2. The top panel shows the CO2 dry air mole fraction, where the grey triangles represent the sample measurements and the black inverted triangles represent the reference measurements. The other three panels show the isotopic composition of the sample gas determined with respect to the reference gas. The error bars denote the 1σ standard deviation of a 60 s measurement. The reference gas is identical to the sample gas, with the only difference being that the sample gas was measured after it had passed through the NAD. The first part of the experiment represents the dry mode (shown with a red background), where, for sample measurements, dry air from a cylinder was passed through a preconditioned NAD and was then introduced into the TILDAS-SICAS for measurements. In the latter half of the experiment in wet mode (shown with a blue background), the dry air form the cylinder was first humidified (∼2 % H2O) and then dried through the NAD before introducing it into the TILDAS-SICAS for measurements. As can be seen, the NAD does not significantly affect the measurements of CO2 mole fractions and its stable isotope composition.
3.4 Effects on other atmospheric trace gases
The performance of the NAD was tested in conjunction with the NOAA-ESRL PCP–PFP sampling system simulating real sampling conditions at the NOAA-ESRL and INSTAAR laboratories in Boulder, Colorado. The objective of these experiments was to test the effect of a known air sample, when collected into the PFP flasks after being dried by the NAD, on CO2 and its two singly substituted isotopologues (13C16O16O and 18O12C16O), as well as on other greenhouse gases measured on the MAGICC system (CH4, CO, N2O and SF6). For the known air sample, we used two different calibrated dry whole air samples in a 29 L Luxfer aluminum cylinder compressed at ∼140 bar. The test gas was flushed and compressed by the PCP into a 12-flask PFP using the setup illustrated in Fig. 5. To provide a homogeneous sample just above atmospheric pressure (∼1.4 kPa) the dry test gas from the cylinder was flushed into a 300 L buffer volume at a rate of 12–13 L min−1. This flow rate was a bit higher than the flow rate of the PCP–PFP during flushing and sample collection, allowing a small excess flow of ∼0.5 L min−1 (measured with a rotameter). A Picarro CRDS was used to measure the stability of CO2, CH4 and H2O in the flow exiting the buffer volume, as shown in Fig. 5. To humidify the air, we used a bubbler containing demineralized water at a laboratory temperature of 23 ∘C, which resulted in a water vapor content of ∼1.4 % (corresponding to ∼40 % relative humidity) by volume as indicated by the Picarro CRDS instrument. To check the relative humidity and temperature of sample air in the buffer volume, a hygrometer (Rotronic HygroPalm HP22-A) was also placed in the excess flow line. The flasks were prepared for sampling by flushing them with dry air, followed by filling them with synthetic air containing ∼350 ppm CO2 at atmospheric pressure. Sampling is started by toggling a switch that initiates the pumps in the PCP and opens valves in the PFP to flush the manifold and flask. After flushing, the flask is pressurized to 275 kPa. We noted that the inclusion of the NAD imposed more drag on the PCP pumping capacity, reducing the flushing flow from ∼15 to ∼12 L min−1. We conducted three successful experiments of filling one 12-flask PFP and used two compressed air cylinders as sample gas during this experimentation period.
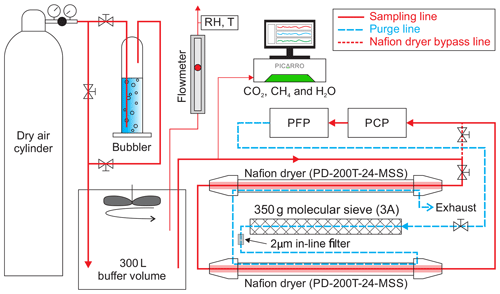
Figure 5Schematic of the setup used at INSTAAR to determine the effect of the NAD on the mole fraction determination of CO, CH4, N2O and SF6 along with CO2 and its singly substituted isotopologues. A NOAA-ESRL PCP–PFP system could be flushed with either dry or humidified cylinder air including or bypassing the NAD. Flow from the cylinder was adjusted such that there was always an overflow of air from the buffer volume, where relative humidity of the sample was constantly monitored. A Picarro analyzer was used to monitor the stability of CO2, CH4 and H2O mole fractions in the gas exiting the buffer volume, during all the tested experimental conditions, a summary of which is shown in Fig. 6.
In these experiments, we tested four different conditions by filling a set of three flasks under the following conditions: (A) dry air without dryer, (B) dry air with dryer, (C) wet air without dryer, and (D) wet air with dryer. When the difference between the base condition and the test condition remained within the WMO recommended compatibility goals (±0.1 and 0.05 ppm for CO2 for the Northern and Southern Hemisphere, respectively; ±2 ppb both for CH4 and CO; ±0.1 ppb for N2O; ±0.02 ppt for SF6; and ±0.03 ‰ for δ13C and ±0.05 ‰ for δ18O; Crotwell and Steinbacher, 2017), we concluded that the test condition did not induce any significant bias to the measurement. With respect to applying these WMO compatibility goals it should be mentioned that these precisions should be seen as the scientifically desirable level of compatibility for concurrent measurements of well-mixed background air by different laboratories, while they may not be the currently achievable best 1σ measurement uncertainty (Crotwell and Steinbacher, 2017). Indeed, a recent study by Zellweger et al. (2019) indicated that the N2O network compatibility goal of 0.1 ppb remains quite challenging to meet even with current state-of-the-art measurement techniques. In Fig. 6 we present the results of five cases comparing a base condition with a test condition in the following order: Case 1: condition B − condition A, Case 2: condition C − condition A, Case 3: condition D − condition A, Case 4: condition D − condition C, and Case 5: condition D − condition B. We show the mean difference and the corresponding 1σ standard deviation (error bar) indicating the spread in the results, while the dashed line indicates the WMO network compatibility goals. Table 1 summarizes the tests and the information we get from each case.
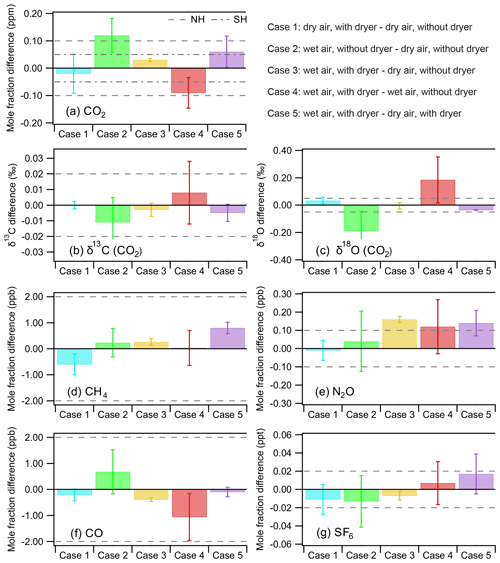
Figure 6Overview of results to determine the effect of the NAD on the mole fraction determination of CO, CH4, N2O and SF6 along with CO2 and isotopic composition of its two singly substituted isotopologues (16O13C16O and 16O12C18O). The dashed lines denote the WMO network compatibility goals for the different species. We note that the compatibility goal for N2O should be seen as a lower limit target value. We present five test cases to illustrate the measurement biases introduced if samples are not sufficiently dried. Case 1 shows the effect of the NAD in dry conditions, whereas cases 3 and 5 show the difference in sampling of wet sample air dried with the NAD before being compressed into the sample flask with respect to dry air sampling. The worst sampling conditions with the largest bias are associated with using humidified sample air (without NAD) shown in cases 2 and 4. When the difference remains within the WMO network compatibility goals, we concluded that the measured difference is not significant (notably for cases 1, 3 and 5 for most species).
Table 1Overview of results from Case 1 to Case 5 (test result – reference value) to determine the effect of the NAD on the mole fraction determination of CO2, CO, CH4, N2O, CO, and SF6, as well as on the isotopic composition of the two singly substituted isotopologues 16O13C16O and 16O12C18O of CO2. The measurement uncertainty denotes the 1σ standard deviation of typically three samples per condition.
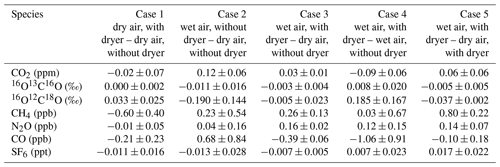
Case 1 (dry air with dryer – dry air without dryer) should indicate if there is any bias introduced by the Nafion™ dryer in the sampling line. Indeed, the results show that the differences for condition B − condition A are all very small and that they stay well within the WMO network compatibility goals. In cases 2 and 4 we compare wet samples (without dryer) containing ∼1.4 % water vapor with either dry air without the dryer or dry air with the dryer, respectively. As expected we find that CO2 and its isotopologues (notably δ18O) are affected. In cases 3 and 5 we compare the wet air with dryer (condition D) with dry air without dryer (condition A) and with dry air with dryer (condition B). These cases prove the benefit of using the NAD by showing that the differences in condition D − condition A and condition D − condition B, notably those of CO2 and its singly substituted isotopologues, are well within the WMO network compatibility goals. Case 5 is in fact comparable to the experiment shown in Fig. 4, only differing in their used flow rates, with the former being a flow-through semicontinuous measurement scheme. Although we have argued that higher flow rates are likely favorable for reduced isotopic exchange (observable in δ18O) due to the reduction in the interaction time between the NAD surface and CO2, Case 5 is slightly more biased than expected, based on Fig. 4. This is likely caused by the additional and variable interaction of the sample with the flask surface, which is not encountered during the flow-through experiment shown in Fig. 4.
Since unbiased measurements of CO2 mole fraction and its isotopic composition in whole air samples demand collection of very dry sample air, we tested and present here the results of a Nafion™-based drying system. Nafion™ dryers are an excellent alternative to chemical and recirculating-chiller-based dryers for mobile sampling platforms. For example, most chemical dryers either alter the chemical composition of the sample air or are considered hazardous from a safety standpoint, especially when they are used on board an aircraft. On the other hand, recirculating-chiller-based dryers are very efficient but are large and extremely energy demanding, which makes their usage on light aircrafts logistically undesirable. Nafion™-based drying systems offer a consumable-free, reusable, and a field-deployable alternative, which does not necessitate incorporating hazardous chemicals and also eliminates the use of any power on board an aircraft. Initial laboratory tests, using the Picarro G2301 analyzer, already indicated that a Nafion™-based system did not alter the mole fraction of CO2 and CH4 in dry and humidified air samples and hence could potentially be a promising alternative. In this work, we tested the NAD which is configured for use with the PCP–PFP system from NOAA-ESRL, although the use of our system is not limited to that sampling platform.
During the development phase we learned that, to achieve the best performance from the dryers in the configuration we wanted to use, they had to be conditioned before every use. Conditioning was performed by flushing the Nafion™ drying tubes and the purge volume overnight with dry air at ∼2 L min−1, and a successful conditioning was achieved when the water content in the dry air entering the dryer was equal to the water content in the air exiting the dryer. Similarly, the molecular sieve granules were also dried following every use by baking them overnight in an oven set at 100 ∘C. Since all the end connectors on the NAD are normally closed Swagelok quick-connect connectors, the system is filled with dry air at ambient pressure and stored. We performed a storage stability check over a period of 1 month, and the results indicated that the NAD, if stored in dry conditions, i.e., filled with dry air immediately after conditioning, would perform similarly to one freshly conditioned. This was concluded by comparing the water removal capacity of the NAD and the lowest achievable water vapor concentration while processing ∼200 L of humidified air (∼2 %) at similar flow rates. This property is particularly beneficial for the sampling conditions in Brazil because the conditioning step is performed in the lab a few days before the PFP and the NAD are shipped to the sample collection site. As such, the application of this drying unit is not only limited to sampling in Brazil, but can also be used in any other situation where drying large volumes of air samples is necessary and availability of electricity is an issue.
In the ASICA program, the goal is to perform measurements of CO2 mole fraction and high-precision measurements of all the singly substituted isotopologues of CO2 to constrain the gross primary production and its response to droughts for the Amazon basin. In this program, 12 samples are collected per flight at altitudes between 300 m and 4.4 km a.m.s.l. To achieve high-precision measurements of the isotopic composition of CO2 in the whole air samples, the collected sample air must be dry. Thus the next requirement was to estimate the water removal capacity of the NAD and estimate the number of flasks that could be filled with a targeted dew point of −2 ∘C (at 275 kPa). From the experiment presented in Fig. 2, it is evident that the response of the NAD is almost instantaneous and approximately 85 L of air, containing 3.7 %–3.9 % H2O, can be processed within the targeted dew point of −2 ∘C. In Fig. 3, we estimated the number of flasks that can be sampled within the targeted dew point temperature during a typical flight above the Amazon. According to these estimates, we can at least collect eight flasks with water vapor content below −2 ∘C dew point temperature (at 275 kPa, 60 % RH), whereas the rest of the flasks would still contain water vapor below 5 ∘C dew point temperature (at 275 kPa, 100 % RH). As discussed earlier, a previous study (Gemery et al., 1996) showed that flask samples containing air with relative humidity below 60 % are generally not affected, and gradual biases are observed in the 60 %–100 % RH range. This would thus indicate that the first eight samples collected between 4.4 and 1.5 km should be free from any bias introduced from the water vapor content in the flask. But the last four samples collected between 1.5 and 0.3 km could be potentially biased towards the isotopic composition of the water it is exposed to, although this bias should be gradual due to the continuous increase of the water vapor content as more samples are processed through the NAD. This prediction, shown in Fig. 3, is of course based on the experiment shown in Fig. 2, where the NAD was directly and continuously exposed to air containing ∼4 % water vapor. This scenario would saturate the NAD much faster, and the ability to dry the Nafion™ tubes through the purge side would be slower, whereas, in a real sampling scenario, the NAD is not exposed to high water vapor content as soon as sampling begins and thus the purge side could still dry the Nafion™ tubes longer than in the former case. This would then lead to an increase in the estimated quantity of water removed by the NAD before crossing the 60 % RH limit (−2 ∘C dew point) in the sampled flasks, as shown in Fig. 3, and would thus encompass more than eight samples within this limit and would also lower the biases for the ones outside the limit.
The next requirement was to establish if the NAD was inert for the gases of interest and did not alter the isotopic composition of CO2 while sampling. To understand the effect of the NAD on the isotopic composition of CO2, we performed a semicontinuous zero-enrichment experiment with the TILDAS-SICAS instrument in our laboratory. In such an experiment, the same gas is treated both as a reference and a sample gas, where the reference stream is unprocessed and the sample stream is processed. Thus, a zero difference between the reference and the sample stream would indicate that the processed gas was not modified at all. This is demonstrated in Fig. 4, where the first part of the experiment shows that the isotopic composition of CO2 is unaltered when dry sample air is passed through the NAD relative to the direct measurement of the dry sample air. The second part of the experiment demonstrates that the isotopic composition of CO2, as observed when wet sample air is passed through the NAD (thus dried) relative to the direct measurement of the dry sample air, remains within the measurement uncertainties and thus indistinguishable. Since the TILDAS-SICAS is not designed for continuous measurements, we performed this experiment at a lower flow rate than what would otherwise be used in field to obtain more discrete measurements while processing a certain volume of air. This demonstrates that, even with a doubling of residence time in the NAD compared to field conditions, the isotopic composition remains unaltered. Therefore, shorter residence times during field measurements would reduce the chances of interaction between CO2 and the wet membrane surface and would therefore be more favorable. Additionally, this experiment also clearly demonstrates that CO2 mole fraction determinations are not significantly affected in the presence of NAD, in both dry and wet modes (sample) when compared to measurements performed without the NAD (reference).
A comprehensive experiment was performed to test the NAD in combination with the PFP–PCP sampling platform at NOAA-ESRL and INSTAAR, where all the atmospheric trace gases were measured as they will be done in Brazil. These experiments were performed in four different conditions and the results are summarized in Fig. 6. The results show that for most species, e.g., CO, CH4, N2O and SF6, the measurements are unaffected when the NAD was used for drying the sampling air and were within or very close, in the case of N2O, to the WMO network compatibility goals. Even in the case of CO2, the mole fraction measurements were not severely affected and stayed within the WMO network compatibility goals. As expected, the isotopic composition of 18O in CO2 was affected in the cases where wet samples were collected relative to dry sample air or wet air dried with the NAD. Additionally, the isotopic composition of 13C in CO2 also remained unaffected in these test conditions.
Through results presented in this paper, we show that the NAD is a viable drying solution and can be used during flight sampling. The NAD, as shown here, does not affect the composition of the whole air samples, with respect to the species described in this paper, and also does not affect the isotopic composition of CO2.
The datasets required to reproduce the figures used in this paper are freely available at https://doi.org/10.34894/VBVVWN (Paul, 2020).
DP, HAS and WP designed the setup with suggestions from JBM, AMC and HAJM. HAS, DP, HGJ, BAMK, SEM performed the experiments. DP and HAS analyzed the data. LG, LD, CC and RL helped in making the NAD compatible with the PCP–PFP system and its deployment in the sampling sites. DP, HAS and WP wrote the paper with input from all the coauthors.
The authors declare that they have no conflict of interest.
We greatly acknowledge the collaboration and kind support of the NOAA Earth System Research Laboratory (ESRL) in Boulder, as well as the Institute of Arctic and Alpine Research (INSTAAR), University of Colorado, Boulder. In particular, we are grateful for both the help and support of Jack Higgs, Don Neff, and Colm Sweeney at NOAA, as well as Bruce Vaugh and colleagues at INSTAAR, for providing their laboratories for our experiments. The authors also acknowledge the European Research Council for funding the ASICA project.
This research has been supported by the European Research Council (ASICA (grant no. 649087)).
This paper was edited by Thomas F. Hanisco and reviewed by two anonymous referees.
Alden, C. B., Miller, J. B., Gatti, L. V., Gloor, M. M., Guan, K., Michalak, A. M., van der Laan-Luijkx, I. T., Touma, D., Andrews, A., Basso, L. S., Correia, C. S., Domingues, L. G., Joiner, J., Krol, M. C., Lyapustin, A. I., Peters, W., Shiga, Y. P., Thoning, K., van der Velde, I. R., van Leeuwen, T. T., Yadav, V., and Diffenbaugh, N. S.: Regional atmospheric CO2 inversion reveals seasonal and geographic differences in Amazon net biome exchange, Glob. Change Biol., 22, 3427–3443, 2016.
Chen, H., Winderlich, J., Gerbig, C., Hoefer, A., Rella, C. W., Crosson, E. R., Van Pelt, A. D., Steinbach, J., Kolle, O., Beck, V., Daube, B. C., Gottlieb, E. W., Chow, V. Y., Santoni, G. W., and Wofsy, S. C.: High-accuracy continuous airborne measurements of greenhouse gases (CO2 and CH4) using the cavity ring-down spectroscopy (CRDS) technique, Atmos. Meas. Tech., 3, 375–386, https://doi.org/10.5194/amt-3-375-2010, 2010.
Conway, T. J., Tans, P. P., Waterman, L. S., Thoning, K. W., Kitzis, D. R., Masarie, K. A., and Zhang, N.: Evidence for interannual variability of the carbon cycle from the National Oceanic and Atmospheric Administration/Climate Monitoring and Diagnostics Laboratory Global Air Sampling Network, J. Geophys. Res., 99, 22831–22855, 1994.
Crosson, E. R.: A cavity ring-down analyzer for measuring atmospheric levels of methane, carbon dioxide, and water vapor, Appl. Phys. B, 92, 403–408, 2008.
Crotwell, A. and Steinbacher, M. (Eds.): GAW Report No. 242, in: 19th WMO/IAEA Meeting on Carbon Dioxide, Other Greenhouse Gases and Related Measurement Techniques (GGMT-2017), Dübendorf, Switzerland, 27–31 August 2017, WMO, 2017.
Dlugokencky, E. J., Steele, L. P., Lang, P. M., and Masarie, K. A.: The growth rate and distribution of atmospheric methane, J. Geophys. Res., 99, 17021–17043, 1994.
Gatti, L. V., Gloor, M., Miller, J. B., Doughty, C. E., Malhi, Y., Domingues, L. G., Basso, L. S., Martinewski, A., Correia, C. S., Borges, V. F., Freitas, S., Braz, R., Anderson, L. O., Rocha, H., Grace, J., Phillips, O. L., and Lloyd, J.: Drought sensitivity of Amazonian carbon balance revealed by atmospheric measurements, Nature, 506, 76–80, 2014.
Gemery, P. A., Trolier, M., and White, J. W. C.: Oxygen isotope exchange between carbon dioxide and water following atmospheric sampling using glass flasks, J. Geophys. Res., 101, 14415–14420, 1996.
Gerbig, C., Schmitgen, S., Kley, D., Volz-Thomas, A., Dewey, K., and Haaks, D.: An improved fast-response vacuum-UV resonance fluorescence CO instrument, J. Geophys. Res., 104, 1699–1704, 1999.
Hoag, K. J., Still, C. J., Fung, I. Y., and Boering, K. A.: Triple oxygen isotope composition of tropospheric carbon dioxide as a tracer of terrestrial gross carbon fluxes, Geophys. Res. Lett., 32, L02802, https://doi.org/10.1029/2004GL021011, 2005.
Hofmann, M. E. G., Horváth, B., Schneider, L., Peters, W., Schützenmeister, K., and Pack, A.: Atmospheric measurements of Δ17O in CO2 in Göttingen, Germany reveal a seasonal cycle driven by biospheric uptake, Geochim. Cosmochim. Ac., 199, 143–163, 2017.
Keeling, R. F., Graven, H. D., Welp, L. R., Resplandy, L., Bi, J., Piper, S. C., Sun, Y., Bollenbacher, A., and Meijer, H. A. J.: Atmospheric evidence for a global secular increase in carbon isotopic discrimination of land photosynthesis, P. Natl. Acad. Sci. USA, 114, 10361–10366, 2017.
Koren, G., Schneider, L., van der Velde, I. R., van Schaik, E., Gromov, S. S., Adnew, G. A., Mrozek, D. J., Hofmann, M. E. G., Liang, M.-C., Mahata, S., Bergamaschi, P., van der Laan-Luijkx, I. T., Krol, M. C., Röckmann, T., and Peters, W.: Global 3D Simulations of the Triple Oxygen Isotope Signature Δ17O in Atmospheric CO2, J. Geophys. Res.-Atmos., 124, 8808–8836, https://doi.org/10.1029/2019jd030387, 2019. 2019.
McManus, J. B., Nelson, D. D., and Zahniser, M. S.: Design and performance of a dual-laser instrument for multiple isotopologues of carbon dioxide and water, Opt. Express, 23, 6569–6586, 2015.
Neubert, R. E. M., Spijkervet, L. L., Schut, J. K., Been, H. A., and Meijer, H. A. J.: A Computer-Controlled Continuous Air Drying and Flask Sampling System, J. Atmos. Ocean. Tech., 21, 651–659, 2004.
Paul, D.: Evaluation of a field-deployable Nafion™-based air drying system for collecting whole air samples and its application to stable isotope measurements of CO2, DataverseNL, V1, https://doi.org/10.34894/VBVVWN, 2020.
Perma-Pure-LLC: https://www.permapure.com/scientific-emissions/resources/all-about-nafion-and-faq/, last access: March 2019.
Peters, W., van der Velde, I. R., van Schaik, E., Miller, J. B., Ciais, P., Duarte, H. F., van der Laan-Luijkx, I. T., van der Molen, M. K., Scholze, M., Schaefer, K., Vidale, P. L., Verhoef, A., Warlind, D., Zhu, D., Tans, P. P., Vaughn, B., and White, J. W. C.: Increased water-use efficiency and reduced CO2 uptake by plants during droughts at a continental-scale, Nat. Geosci., 11, 744–748, 2018.
Sakai, S., Matsuda, S., Hikida, T., Shimono, A., McManus, J. B., Zahniser, M., Nelson, D., Dettman, D. L., Yang, D., and Ohkouchi, N.: High-Precision Simultaneous 18O∕16O, 13C∕12C, and 17O∕16O Analyses for Microgram Quantities of CaCO3 by Tunable Infrared Laser Absorption Spectroscopy, Anal. Chem., 89, 11846–11852, 2017.
Soundings-Data: University of Wyoming, Upperair Air Data: Soundings, available at: http://weather.uwyo.edu/upperair/sounding.html, last access: April 2019.
Stephens, B. B., Miles, N. L., Richardson, S. J., Watt, A. S., and Davis, K. J.: Atmospheric CO2 monitoring with single-cell NDIR-based analyzers, Atmos. Meas. Tech., 4, 2737–2748, https://doi.org/10.5194/amt-4-2737-2011, 2011.
Sweeney, C., Karion, A., Wolter, S., Newberger, T., Guenther, D., Higgs, J. A., Andrews, A. E., Lang, P. M., Neff, D., Dlugokencky, E., Miller, J. B., Montzka, S. A., Miller, B. R., Masarie, K. A., Biraud, S. C., Novelli, P. C., Crotwell, M., Crotwell, A. M., Thoning, K., and Tans, P. P.: Seasonal climatology of CO2 across North America from aircraft measurements in the NOAA/ESRL Global Greenhouse Gas Reference Network, J. Geophys. Res.-Atmos., 120, 5155–5190, 2015.
Trolier, M., White, J. W. C., Tans, P. P., Masarie, K. A., and Gemery, P. A.: Monitoring the isotopic composition of atmospheric CO2: Measurements from the NOAA Global Air Sampling Network, J. Geophys. Res., 101, 25897–25916, 1996.
van der Laan, S., Neubert, R. E. M., and Meijer, H. A. J.: A single gas chromatograph for accurate atmospheric mixing ratio measurements of CO2, CH4, N2O, SF6 and CO, Atmos. Meas. Tech., 2, 549–559, https://doi.org/10.5194/amt-2-549-2009, 2009.
van der Laan-Luijkx, I. T., van der Velde, I. R., Krol, M. C., Gatti, L. V., Domingues, L. G., Correia, C. S. C., Miller, J. B., Gloor, M., van Leeuwen, T. T., Kaiser, J. W., Wiedinmyer, C., Basu, S., Clerbaux, C., and Peters, W.: Response of the Amazon carbon balance to the 2010 drought derived with CarbonTracker South America, Global Biogeochem. Cy., 29, 1092–1108, 2015.
Vaughn, B., Miller, J., Ferretti, D. F., and White, J. W. C.: Stable isotope measurements of atmospheric CO2 and CH4, Elsevier B.V., 2004.
Welp, L. R., Keeling, R. F., Weiss, R. F., Paplawsky, W., and Heckman, S.: Design and performance of a Nafion dryer for continuous operation at CO2 and CH4 air monitoring sites, Atmos. Meas. Tech., 6, 1217–1226, https://doi.org/10.5194/amt-6-1217-2013, 2013.
Wright, I. P., McNaughton, N. J., Fallick, A. E., Gardiner, L. R., and Pillinger, C. T.: A high-precision mass spectrometer for stable carbon isotope analysis at the nanogram level, J. Phys. E, 16, 497–504, 1983.
Zellweger, C., Steinbrecher, R., Laurent, O., Lee, H., Kim, S., Emmenegger, L., Steinbacher, M., and Buchmann, B.: Recent advances in measurement techniques for atmospheric carbon monoxide and nitrous oxide observations, Atmos. Meas. Tech., 12, 5863–5878, https://doi.org/10.5194/amt-12-5863-2019, 2019.





