the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Uptake behavior of polycyclic aromatic compounds during field calibrations of the XAD-based passive air sampler across seasons and locations
Yuening Li
Yushan Su
Ying Duan Lei
Chubashini Shunthirasingham
Zilin Zhou
Jonathan P. D. Abbatt
Hayley Hung
Polycyclic aromatic compounds (PACs) continue to demand attention due to their widespread presence and well-established health implications. Given that incomplete combustion is a major contributor to PACs and inhalation constitutes a crucial human exposure pathway, a comprehensive understanding of the concentrations, spatial distributions, and fates of a broad range of PACs in the atmosphere is important. Passive air samplers (PASs) are a commonly utilized technique for PAC sampling and monitoring. In this study, we present the results from two 1-year calibration experiments, one starting in summer and the other in winter, using a passive air sampler equipped with XAD resin as the sorbent (XAD-PAS). Throughout both experiments, PACs were consistently sorbed during the initial 6-month period. However, the sorbed amounts for many PACs exhibited a decrease after half a year of deployment. Three hypotheses to explain this phenomenon were explored, including the uptake of atmospheric particles, evaporation from the sorbent, and reactions with photo-oxidants. All had to be rejected based on the obtained data, additional laboratory experiments, and model results. Model simulations were further used to (i) confirm that a loss process must be invoked to explain the observed uptake behavior and (ii) estimate the kinetics of that loss process for different PACs. Sampling rates (SRs) for 28 PACs derived from the linearized uptake curves during the first 6 months of deployment were comparable to those of other semi-volatile organic compounds obtained during the same calibration experiment, and they also demonstrate a consistent negative correlation with volatility.
- Article
(880 KB) - Full-text XML
-
Supplement
(2711 KB) - BibTeX
- EndNote
Approximately 4 million km2 of savanna, forest, grassland, and agricultural ecosystems are burned through both natural and controlled fires annually (Nolan et al., 2022). These fire events have attracted global attention to the release of pollutants, including harmful particles, organic vapors, and greenhouse gases, into the air as well as the related potential health risks. In particular, the production and dispersion of polycyclic aromatic compounds (PACs) in the environment have emerged as significant concerns. PACs comprise organic molecules composed of fused aromatic rings, including unsubstituted polycyclic aromatic hydrocarbons (PAHs), alkylated PAHs (alk-PAHs), and heterocyclic aromatic compounds containing N, O, or S atoms in their structure (Moradi et al., 2022). PACs originate from a multitude of sources, encompassing both natural and human-related activities, many related to the incomplete combustion of organic matter. Natural sources include wildfires (Nolan et al., 2022; Environment Canada and Health Canada, 1994; Nikolaou et al., 1984; Wnorowski et al., 2021), volcanic eruptions (Nikolaou et al., 1984; United Nations Environment Programme, 2020), and biogenic processes (Wakeham et al., 1980). Anthropogenic activities substantially contribute to the emissions of PACs into the environment (Environment Canada and Health Canada, 1994), with vehicle emissions (Berthiaume et al., 2021; Muir and Galarneau, 2021), cooking and heating (Shen et al., 2012; Environment Canada and Health Canada, 1994), wood burning (Lima et al., 2005; Xu et al., 2006), industrial processes (Lima et al., 2005; Xu et al., 2006), and tobacco smoke (Holme et al., 2022) releasing numerous PACs into the atmosphere. PAHs have been widely studied due to their ubiquitous presence in the environment and their potential for eliciting adverse health effects, such as allergic potential, carcinogenicity, teratogenicity, and genotoxicity (Boffetta et al., 1997; White, 2002; Kim et al., 2013; World Health Organization, 1998; ATSDR, 1995), PAHs are regulated and routinely monitored by numerous agencies and governments across the globe.
With past research efforts mainly focused on PAHs, alk-PAHs have only recently started to garner attention from researchers. The addition of alkyl groups alters the physicochemical properties of PAHs, affecting their solubility, volatility, and toxicological properties. Certain alk-PAHs may be more toxic than their non-alkylated counterparts (Golzadeh et al., 2021; Hawthorne et al., 2006; Andersson and Achten, 2015; Grung et al., 2011; Kaisarevic et al., 2009; Pcchillips et al., 1979; Sarma et al., 2017), raising concerns about their potential ecological and human health impacts. Whereas alk-PAHs have been identified at elevated concentrations and in some cases are even dominant among PACs in air (Wnorowski et al., 2022; Moradi et al., 2022; Jariyasopit et al., 2019) and food (Golzadeh et al., 2021), they have not been widely studied, monitored, and regulated (Moradi et al., 2022). Studies in Canada are particularly limited in number, with some data available for the Greater Toronto Area (Jariyasopit et al., 2019; Moradi et al., 2022) and Athabasca oil sands region (Harner et al., 2013; Jariyasopit et al., 2018; Wnorowski et al., 2021; Mahoney et al., 2023; Cheng et al., 2018; Rauert et al., 2020; Harner et al., 2018; Moradi et al., 2023; Jariyasopit et al., 2021; Ahad et al., 2021).
Because inhalation is a critical human exposure pathway (Boström et al., 2002; Liu et al., 2007), achieving a comprehensive understanding of PAC concentrations, spatial distribution, and fate in the atmosphere is important. As atmospheric PAC concentrations are related to proximity to emission sources, urban areas, industrial zones, regions with high traffic density, and places close to wildfire typically exhibit elevated PAC concentrations. Atmospheric transport can disperse PACs widely, both while bound to particles and in the gas phase (Muir and Galarneau, 2021; Zhou et al., 2019; Wnorowski et al., 2022; Masclet et al., 2000). Clearly, there is a need for reliable air sampling techniques for a wide range of PACs.
Atmospheric PACs can be sampled using active air samplers (AASs) and passive air samplers (PASs). In AASs, pumps are used to pull air through a sampling medium (e.g., a sorbent and/or a filter) to capture atmospheric PACs in the gas and/or particle phase. While accurate sampling volumes are usually easily obtained, the need for a stable electrical power supply and high maintenance requirements as well as operational expenses limit the geographical scope of AASs, especially in remote areas. Without using pumps, PASs sample and retain chemicals by relying on chemical vapors diffusing and sorbing to a sorbent. Low cost and maintenance requirements expand their potential spatial applications, e.g., in areas close to wildfire regions. However, obtaining accurate sampling volumes can be challenging. Confidently using a PAS requires quantitative knowledge of the uptake kinetics and of the limits of linear uptake for the targeted compounds, which is typically obtained by calibration studies using co-located AASs and PASs.
Sorbents used for sampling PACs in PASs include polyurethane foam (PUF) (Cheng et al., 2013; Domínguez-Morueco et al., 2017; Pozo et al., 2015), polyethylene (PE) (Bartkow et al., 2004; Meierdierks et al., 2021), polydimethyl siloxane (PDMS) (Barthel et al., 2012; Bohlin-Nizzetto et al., 2020), and styrene−divinylbenzene co-polymeric resin (commercial name XAD) (Barthel et al., 2012; Lévy et al., 2018). Calibration studies have been conducted for PACs in PAS based on PE (Meierdierks et al., 2021), PUF (Harner et al., 2013; Bohlin-Nizzetto et al., 2020; Tromp et al., 2019; Holt et al., 2017; Bohlin et al., 2014a; Melymuk et al., 2011), PDMS (Tromp et al., 2019), and XAD (Ellickson et al., 2017; Armitage et al., 2013). However, due to a relative low uptake capacity, some sorbents, such as PE and PDMS, are less widely used in PAS. Even though PUF-PASs are widely used, they have some limitations: (1) the uptake capacity of PUF is too small to sample more volatile PACs within the linear uptake regime during longer deployment periods, which may lead to relatively high uncertainties and difficulties in data interpretation (Li and Wania, 2021; Li et al., 2022). (2) Gas-phase concentrations of PACs, especially of less volatile PACs, may be hard to obtain as the PUF-PAS samples both gaseous and particle-bound PACs (Melymuk et al., 2011), with sampling rates for the latter suffering from high variability and uncertainty (Holt et al., 2017).
The significantly greater uptake capacity (Wania and Shunthirasingham, 2020; Hayward et al., 2011) of the XAD-PAS (Wania et al., 2003) results in a demonstrably longer linear uptake period (Wania and Shunthirasingham, 2020; Li et al., 2023a, b) for many semi-volatile organic compounds (SVOCs) compared to other PASs. Moreover, the XAD-PAS's shelter was intentionally designed to mitigate the impact of wind and particle uptake. Although the XAD-PAS has been calibrated for PACs twice (Ellickson et al., 2017; Armitage et al., 2013), these studies only targeted a limited number of PAHs and no alk-PAHs or heterocyclic aromatic compounds. While Armitage et al. (2013) deployed AASs and PASs side-by-side, Ellickson et al. (2017) positioned certain PASs at a considerable distance from the AASs, which could potentially introduce heightened levels of uncertainty into the findings. The current study sought to conduct a “gold standard” calibration for XAD-PAS with the following objectives: (1) evaluate the uptake behaviors of a large number of PACs in XAD-PAS, including PAHs, alk-PAHs, and the heterocyclic aromatic compound dibenzothiophene; (2) determine the linear uptake regimes and obtain experimental sampling rates for these PACs; and (3) assess the impact of meteorological conditions and chemical properties on the sampling of these PACs.
2.1 Field sampling
The main calibration experiment was conducted in 2020–2021 on the campus of the University of Toronto Scarborough in the eastern suburbs of Toronto (43.7837, −79.1903), with results for other SVOCs reported previously (Li et al., 2023a, b). For comparison, we also present the results of calibration experiments conducted in 2001–2002 in a forest (44.3184, −79.9341) and a nearby clearing (44.3270, −79.9169) site in Borden, Ontario, with air concentrations having been previously reported (Su et al., 2007a, b). The Borden sites are approximately 85 km to the northwest of the Toronto site. In each study, XAD-PASs were deployed simultaneously ca. 1.5 m above the ground, and AASs, collecting particle and gas phase separately, were placed side by side with the PASs. Both calibration experiments lasted ∼1 year, with the Borden experiments starting in November 2001 and the Toronto experiment in June 2020. During the experiments, XAD-PASs were retrieved every 4 weeks in Toronto and after 36, 60, 120, 181, 246, 323, and 365 d in Borden. Periodical 24 h active air samples in Borden and consecutive week-long active air samples in Toronto were obtained using high-volume active air samplers (HV-AASs) and a mid-volume active air sampler (MV-AAS), respectively. Detailed sampling information has been described in previous publications (Su et al., 2007a, b; Li et al., 2023a, b).
2.2 Sample treatment
Glass-fiber filters (GFFs) and PUFs from the Borden experiment were Soxhlet-extracted with dichloromethane and petroleum ether, respectively. Extracts were cleaned, fractionated, and concentrated as described previously (Su et al., 2006, 2007a). The XAD from the PASs deployed at Borden was loaded to and extracted in an elution column with 250 mL of methanol, followed by 350 mL of dichloromethane. After removing methanol with 250 mL of 3 % sodium chloride, extracts were concentrated and fractionated using the method described in Wania et al. (2003). The fractionated extracts were solvent-exchanged into iso-octane and finally concentrated to 0.5 mL using nitrogen. More detail on sample treatment is available in earlier publications (Su et al., 2006, 2007a; Wania et al., 2003).
For the samples from the Toronto experiment, one XAD-PAS cylinder from each retrieval date, all blank samples, the PUF-XAD-PUF sandwiches, and GFFs were subjected to extraction using an accelerated solvent extractor (Dionex 350). Prior to extraction, labeled standards (Table S2) were spiked onto the samples as surrogates. The extracts were then concentrated using rotary evaporation; water residues were removed using sodium sulfate columns, solvent-exchanged into iso-octane, and further concentrated to 0.1 mL for PAS and 0.5 mL for AAS using nitrogen blowdown. Prior to instrumental analysis, 2.96 and 14.8 ng injection standards (Table S2) were added to PAS and AAS extracts, respectively. Detailed procedures were reported previously (Li et al., 2023a, b).
2.3 Instrumental analysis
Extracts from the Borden experiment were analyzed for 16 PAHs with an Agilent 6890 gas chromatograph (GC) equipped with an Agilent 5973 mass spectrometric (MS) detector using electron ionization in selected ion monitoring mode. In extracts from the Toronto experiment, a total of 22 PAHs, 22 alk-PAHs, and dibenzothiophene were analyzed. This analysis was carried out using an Agilent 7890A GC coupled with a 7000A triple quadrupole MS for PAS and PUF/XAD sandwich extracts and an Agilent 8890 GC coupled with a 7010B MS/MS for the GFF extracts, using electron ionization in multiple reaction monitoring mode. Details on instruments, columns, temperatures, and other parameters are given in the Supplement.
2.4 Ozone exposure experiment
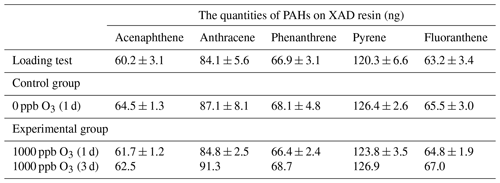
A laboratory experiment was conducted to examine the potential of PAHs to undergo reactions with ozone while being sorbed to XAD. Approximately 150 g of pre-cleaned XAD-2 resin was added to ∼300 mL of an acetone solution containing ∼3500–5000 ng each of acenaphthene, anthracene, phenanthrene, pyrene, and fluoranthene. To make sure the XAD resin was well mixed, it was stirred continuously using a clean spatula until all acetone had evaporated (confirmed by repeatedly weighing of the PAC-loaded XAD resin), and ∼5.0 g of the spiked XAD resin was added to 10 mesh cylinders (2.0 cm diameter), as used in the XAD-PAS, reducing the amount of resin required by placing a smaller empty mesh cylinder (1.0 cm diameter) in their center (Zhang et al., 2011). The exact weight of the XAD in each mesh cylinder was recorded and used for data normalization.
Five randomly selected spiked mesh cylinders were extracted and analyzed using the same method as for the samples from the Toronto experiment mentioned above in order to investigate the variability of the PAHs in these cylinders. Three mesh cylinders were exposed to ∼1000 ppb ozone at 0 % relative humidity (RH) in a flow tube (Zhou and Abbatt, 2021) (Fig. S1 in the Supplement), two for 24 h and one for 72 h. As a control, two mesh cylinders were exposed to air for 24 h at 0 % RH in the same flow tube. The volume of the flow tube was ∼1.8 L and the flow rate inside the tube was 1800 cm3 min−1. Upon retrieval, the five mesh cylinders from the flow tube experiments were extracted and analyzed as before.
2.5 Calculation of sampling rates (SRs)
During the linear uptake phase, the SR of a compound in the XAD-PAS is linearly related to the effective sampling volume Veff (m3):
where MPAS (ng) is the field-blank-corrected amount of a PAC sorbed to the XAD-2 resin, and Cair is the atmospheric gas-phase concentration of the PAC (ng m−3) averaged over the deployment period t of a sampler (day). Cair is derived by averaging the concentrations recorded by the AAS during a PAS's deployment. The SR for each compound during the period of linear uptake is then obtained as the slope of the linear regression between Veff and t.
2.6 Chemical property compilation
The logarithm of the equilibrium concentration ratios between XAD resin and air (KXAD/air, L air g−1 XAD) at 20 ∘C (Table S4) and the internal energies of phase transfer between XAD and the gas-phase ΔUXAD/air (J mol−1, Table S4) were calculated using solute descriptors for PACs (UFZ-LSER, 2022) and the poly parameter free energy relationships (ppLFERs) by Hayward et al. (2011). The log KXAD/air values at the mean temperatures over the entire deployment period of the Borden and Toronto calibration experiments (7.9 and 12.2 ∘C, respectively) (Table 2) were obtained from log KXAD/air at 20 ∘C and ΔUXAD/air using the van't Hoff equation (Atkinson and Curthoys, 1978; Goss, 1996).
2.7 Model simulations
A mechanistic model developed by Zhang and Wania (2012) was used to simulate the uptake of PACs with different KXAD/air during the two calibration experiments beginning in different seasons (June and November). This model describes the diffusion of chemicals from the atmospheric gas phase to the sorbent of a PAS and the kinetics of reversible sorption to that sorbent. It was modified to consider degradation loss of a chemical sorbed to the XAD resin and to incorporate time-variant temperature and atmospheric ozone concentrations. The Levenberg–Marquardt algorithm was used to find the combinations of sorption rate ksorb and degradative loss rate kd that provide the best fit between predicted and measured uptake curves. The actual temperatures measured during the two calibration experiments (Figs. S2 and S3) were used in the simulations. Measured ozone concentrations reported for the vicinity of the sampling sites (Figs. S4 and S5) were used as input for photo-oxidant degradation simulations only. The thickness of the stagnant air boundary layer was assumed to be 0.01 cm (Zhang and Wania, 2012; Li et al., 2023b) and the simulated deployment length was 336 d.
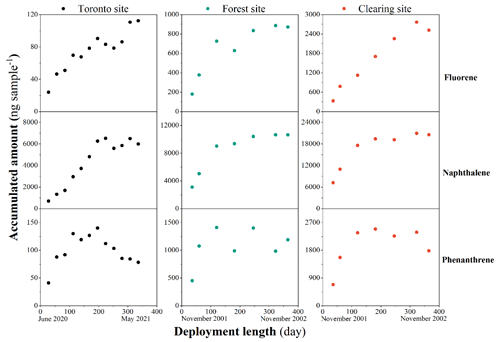
Figure 1The quantities of three representative chemicals accumulating in the XAD-PASs throughout the 1-year deployment period. The black, green, and red markers indicate the blank-corrected measured values at the site in Toronto as well as the forest and clearing sites in Borden, respectively. The deployment month and the month in which the last PAS was retrieved are also indicated at the bottom part of this figure.
3.1 Amounts accumulated in PASs
A total of 15 PAHs, 12 alk-PAHs, and dibenzothiophene were reliably detected in the PAS extracts from the Toronto experiment (Table S6). Seven of these PAHs were also detected in the extracts from the Borden experiment (Table S7). The amounts of fluorene, naphthalene, and phenanthrene accumulated in the PASs are plotted versus deployment length in Fig. 1. Plots for the remaining 25 PACs are provided in Figs. S6 and S7 in the Supplement. These plots reveal that only for five of the 28 PACs in the Toronto experiment, namely acenaphthylene, fluorene, 1-methylfluorene, chrysene, and benzo[b]fluoranthene, does the amount sorbed to the XAD increase relatively continuously with increasing deployment time for the entire length of the experiment. The quantities of the remaining PACs were either largely constant (e.g., naphthalene in Fig. 1) or decreased (e.g., phenanthrene in Fig. 1) after approximately 6 months of deployment. The observed uptake behavior was consistent among the three study sites, i.e., was observed in Toronto and the forest and clearing sites in Borden (Fig. 1). This behavior is in contrast to continuous uptake observed for a large number of SVOCs targeted in the same calibration study in Toronto (Li et al., 2023a, b).
Theoretically, the amount of a chemical taken up in a PAS should always increase with deployment length (Wania and Shunthirasingham, 2020). A decrease with increasing deployment length might occur if the chemical is degraded while being sorbed or if the rate of evaporation from the sorbent exceeds the rate of uptake. The latter occurs only if a chemical's air concentration decreases or temperature increases after the chemical has reached a state of equilibrium between atmospheric gas phase and sorbent. Equilibrium is more easily established for volatile chemicals, sorbents with low uptake capacity, and long deployments. We explored three hypotheses to explain these unexpected decreasing trends in the amounts accumulated in PASs.
3.2 Can uptake of particle-bound compounds explain decreasing trends in the amounts accumulated in PASs?
The first hypothesis posits that the observed uptake behavior was caused by the uptake and accumulation of atmospheric particles in the XAD-PAS. This hypothesis is based on previous studies showing that some PASs do not efficiently block wind from carrying particle-bound substances to the sorbent, as is, e.g., observed in the case of the PUF-PAS with double bowl shelters (Wania and Shunthirasingham, 2020; Chaemfa et al., 2009; Bohlin et al., 2014b; Harner et al., 2013). Even though there is currently no evidence of the uptake of particle-bound SVOCs in the XAD-PAS, if such uptake were to occur, the accumulated amount of a chemical in the XAD-PAS would be the sum of the amount taken up from the gas phase and the amount derived from sampled particle phase. While this admittedly cannot explain why the amount of a chemical quantified in the sorbent would decrease with deployment time, it could result in inconsistent and unpredictable uptake of PACs because such uptake would be influenced by the size, type, and concentrations of particles in air and the wind exposure of a PAS during deployment. Some of such inconsistent uptake could conceivably be suggestive of compound loss from the sampler sorbent.
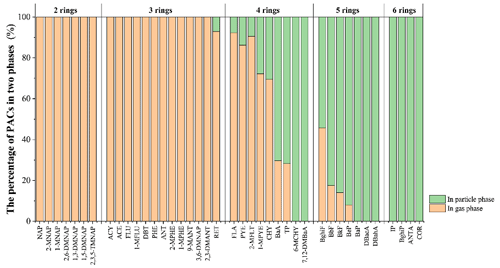
Figure 2The average percentages of PACs detected in the particle (green) and gas phase (orange) of the 48-week-long active air samples taken during the Toronto experiment. PACs were grouped based on the number of rings in their structure.
A total of 17 PAHs and four alk-PAHs were reliably detected in the particles collected on the GFFs from the AAS in the Toronto experiment. Almost all two- and three-ring PACs were only detected in the gas phase; some four-ring and five-ring PACs could be detected in both the gas and particle phase, and nine less volatile PACs were only found in the particle phase (Fig. 2). This is consistent with previous studies (Lewis and Coutant, 2020; Ravindra et al., 2008; Terzi and Samara, 2004) indicating that less volatile PACs with four or more rings are mainly associated with atmospheric particles, whereas volatile PACs are mostly in the gas phase.
Only PACs with at least four or more fused rings in their structure could be detected with a high percentage in the particle phase, whereas the chemicals taken up by the XAD-PASs were primarily more volatile PACs with three or fewer rings. The absence of these relatively volatile PACs in the particle phase (i.e., levels below the limit of detection) demonstrates that the variation of their accumulated amount in XAD-PASs in the second half of the year-long deployment cannot be associated with the uptake of atmospheric particles. Because concentrations of particle-bound PACs in Ontario are much higher in winter than in summer (Su et al., 2007a), their hypothetical uptake would be expected to occur during the latter half of the Toronto experiment and the first half of the Borden study. However, the same deviations from continuous uptake are apparent in the second half of both experiments (Fig. 1).
In the case of the least volatile PACs with five and six rings that were only detected in the particle phase, their absence in PAS extracts provides further evidence that the XAD-PAS does not take up atmospheric particles. Daly et al. (2007) previously reported that involatile chemicals tend to have levels below the limit of detection in XAD-PAS extracts, which is also consistent with our previous studies indicating that no SVOCs with log KXAD/air higher than 7.0 at 12.2 ∘C were detected in XAD-PASs (Li et al., 2023a, b). All evidence therefore refutes the first hypothesis that particle uptake may be the cause of the variation in the uptake amounts.
3.3 Can a limited sorbent uptake capacity explain decreasing trends in the amounts accumulated in PASs?
The second hypothesis is that the uptake capacity for XAD-PAS is too small to prevent some PACs from reaching equilibrium between the sorbent and atmospheric gas phase after 6 months of deployment. A subsequent decrease in sorbed amount could then be attributed to a decrease in air concentration or an increase in temperature, both of which would allow evaporative loss to exceed uptake. A variant of this hypothesis is that something is causing a decrease in the sorption capacity of the XAD resin over time, i.e., the aging of the resin or competition by other sorbates. Several pieces of evidence do not support this interpretation.
During the same calibration experiment in Toronto, we observed highly linear uptake over the entire 48 weeks for SVOCs as volatile as hexachlorobutadiene and monochlorinated biphenyl, with estimated log (KXAD/air/(L air g−1 XAD)) values of 2.95 and 3.56 at 20 ∘C, respectively. Naphthalene, the most volatile of the PACs targeted, has an estimated log (KXAD/air/(L air g−1 XAD)) of 2.69. It is implausible that some chemicals remain in the linear uptake phase, while chemicals with a very similar or higher affinity for sorbing to XAD from the gas phase would reach equilibrium in the same experiment. Furthermore, there is no indication that the extent of loss of PAC from the PAS during the second half of deployment is related to compound volatility. For example, whereas phenanthrene is less volatile than either naphthalene or fluorene, i.e., has a higher KXAD/air (Table 2), it was lost from the XAD-PAS to a greater extent than either of these PACs (Fig. 1).
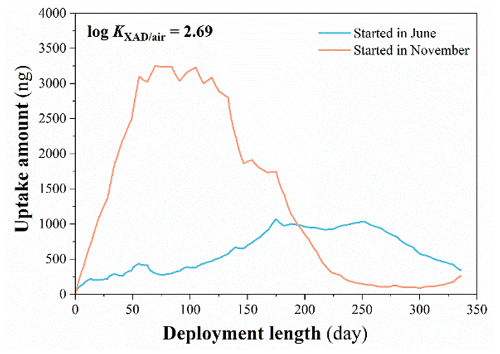
Figure 3Predicted uptake curves for a volatile chemical reaching equilibrium between XAD and the gas phase, i.e., with a log (KXAD/air/(L air g−1 XAD) value of 2.69 at 20 ∘C, during calibration experiments starting in June and November, while assuming air concentration and temperature variability typical for southern Ontario. The thickness of the stagnant air boundary layer was set to 0.01 cm, the sorption rate was set as 3×105 d−1, and no degradation was assumed.
The potential for evaporative net loss of a PAC from the XAD-PAS is largest in summer, when PAC air concentrations tend to be lower and temperatures are highest. This implies that this potential for evaporative loss during the second half of a 1-year experiment would be higher in the case of the Borden calibrations (starting in November) than in the Toronto experiment (starting in June). Figure 3 displays simulated uptake curves for a compound that is volatile enough to reach equilibrium between XAD and the gas phase. Applying the seasonal variability of naphthalene air concentrations and the temperatures measured in Borden and Toronto (Fig. S7), the model predicts widely divergent uptake curves for deployments starting in November and June. The loss of sorbed naphthalene occurs earlier during an experiment starting in winter (after ca. 120 d of deployment), and the extent of loss is more pronounced. In contrast, the naphthalene uptake curves observed in the field calibration experiments are remarkably similar (Fig. 1). In summary, all evidence indicates that PACs did not reach equilibrium and a limited XAD uptake capacity cannot explain the absence of continuous uptake.
3.4 Can reactions with photo-oxidants explain decreasing trends in the amounts accumulated in PASs?
The third hypothesis posits that reactions of sorbed PACs with photo-oxidants present in the atmosphere could account for the lack of continuous uptake (Jariyasopit et al., 2015; Melymuk et al., 2017). It is well established that ozone can react with PAHs sorbed to solid phases (Borrowman et al., 2016; Zhou et al., 2019) and atmospheric particles (Van Vaeck and Van Cauwenberghe, 1984; Kasumba and Holmén, 2018). While nitrogen dioxide may also react with some PAHs, either no reactions were found (Grosjean et al., 1983; Pitts et al., 1980), or only negligible percentages of PAHs were observed to react with nitrogen dioxide (Tokiwa et al., 1981). Even though gaseous and sorbed PAHs can react with OH radicals (Brubaker and Hites, 1998; Atkinson and Arey, 2007; Esteve et al., 2004, 2006; Bedjanian et al., 2010), such reactions are less likely to occur within the dark environment of a XAD-PAS housing because of the short atmospheric lifetime of OH radicals. Consequently, we focused solely on the reaction between ozone and PACs.
Specifically, we exposed PAHs sorbed to XAD to high concentrations of ozone in a flow tube to probe the possibility of degradative loss. If the reaction rate of the PACs with ozone is assumed to be proportional to the ozone concentration, an exposure to ∼1000 ppb of ozone for 24 and 72 h is equivalent to 2 to 4 months of continuous exposure to atmospherically relevant ozone levels of ∼25 ppb. During the sampling period in Toronto, the ozone concentration measured at an air monitoring station (43.7479, −79.2741) located ca. 8 km from our sampling site ranged from 10 to 50 ppb with an annual average of 26 ppb (Fig. S4). The ozone concentration in the vicinity of the Borden site had a similar range (10 to 60 ppb) and mean (27 ppb) (Fig. S5).
We succeeded in obtaining a consistent loading of PAHs in the XAD-filled mesh cylinders: the quantities of five PAHs on the XAD, when normalized to the net weight of resin in a mesh cylinder, had a relative standard deviation (RSD) between 4.6 % and 6.6 % (Table 1). Neither extended exposure to ozone in the flow tube nor exposure to air in the control experiment resulted in a significant change in these quantities of PAHs in the XAD-filled mesh cylinders (p>0.05) (Table 1). In other words, the experiments suggest that ozone did not react with the PAHs sorbed to XAD resin. Interestingly, even though the wall loss of ozone in the flow tube is negligible (Zhou and Abbatt, 2021), we did observe the continuous loss of ozone during the flow tube experiments (Fig. S8). This loss was much larger than when no XAD was present in the flow tube, i.e., can be attributed to the resin. One potential explanation is that ozone reacts with the benzene rings in the XAD resin, and, because of the orders of magnitude higher abundance of these aromatic structures compared with the sorbed PAHs, this reaction may protect the sorbed PAHs from being attacked.
Further evidence that reactions with photo-oxidants are not responsible for the loss of PACs from the XAD resin is again provided by the expected seasonal variability in the importance of that process. In both Toronto and Borden, the ozone concentrations are seasonally variable with higher levels in late summer and early fall and lower levels in winter (Figs. S4 and S5). It should thus affect the second half of a 1-year deployment much more strongly in the Borden experiment than the Toronto experiment. No such difference is apparent. To simulate the influence of ozone on the uptake amount of a PAC, a model simulation was conducted for three representative PACs in the Toronto experiment using the model by Zhang and Wania (2012) modified to allow the sorbed PAC to react with atmospheric ozone. The results from simulations show that the R2 values of the best-fitting uptake curves for these three PACs decrease when the actual time-variant ozone concentration is used as an input parameter (Fig. S9). Finally, we note that PAHs that are known to have a higher reactivity with ozone than others, such as fluorene (Kasumba and Holmén, 2018), anthracene (Kasumba and Holmén, 2018), and benz[a]anthracene (Van Vaeck and Van Cauwenberghe, 1984), do not appear to show a higher rate of loss from the XAD-PAS when compared to the other PACs. Consequently, based on these discussions and analyses, we conclude that ozone was not the cause of the decreasing trend of these PACs in the XAD-PASs.
3.5 What can explain decreasing trends in the amounts accumulated in PASs?
After rejecting all three of our hypotheses, we can formulate a number of constraints on any further potential explanations. We find that the phenomenon is affecting different PACs to a different extent, but this extent is related neither to the PACs' volatility, e.g., as expressed through the KXAD/air, nor their relative reactivity with photo-oxidants. We further find that the phenomenon appears to be very similar in calibration studies started in early winter and early summer in a region with a strongly seasonal climate. This suggests that the strength of the process causing this phenomenon cannot be strongly affected by season. All three of our hypotheses relied on a seasonally variable process: PAC particle concentrations peak in winter, temperature and therefore evaporative loss potential is highest in summer, and photo-oxidant concentrations and degradative loss potential are also highest in summer.
We used additional model simulations (Zhang and Wania, 2012) to further shed light on this issue. For 18 PACs we determined the combination of ksorb and kd values that resulted in the best fit between model-predicted uptake curves and the uptake curves measured during the Toronto experiment (R2>0.4). The fits are shown in Fig. S10 and the fitted parameters are summarized in Table S10. The ksorb values range between 2500 and 300 000 d−1, with higher values for more volatile PACs, as observed previously (Li et al., 2023b). This range encompasses the range of ksorb values previously estimated for polychlorinated biphenyls (10 000 to 80 000 d−1) (Li et al., 2023b). The kd values range from extremely low values for benzo[b]fluoranthene to 0.073 d−1 for triphenylene, the latter corresponding to a half-life on the order of 9 d. For many of the PACs, the fitted kd values indicate a loss process with a half-life ranging from 10 d (2-methylfluoanthene) to 100 d (phenanthrene). We further explored whether it is possible to obtain model results that describe the observation if it is assumed that the sorbed PACs do not undergo loss. Uptake curves predicted using the ksorb values in Table S10 and a kd of zero, i.e., without degradative loss, deviate strongly from the measured ones (Fig. 11). In other words, the model cannot find a best fit to the observations without a loss process.
In summary, the evidence suggests the presence of a process that results in the loss of PACs from the XAD resin with half-lives on the order of weeks to months. The kinetics of that loss process are different for different PACs, with the kd values in Table S10 approximating the relative susceptibility of different PACs. We have to concede that we presently do not know the nature of that loss process. Microbial degradation could be a possibility, although reported relative rates of degradation for PAHs (Ghosal et al., 2016) are not consistent with our kd values; i.e., PAHs with shorter biodegradation half-lives do not appear to show a higher rate of loss from the XAD-PAS. Microbial degradation is likely to be very complex and potentially related to microbial species and communities, as well as environment conditions. More efforts are needed in future studies to further identify the process leading to the loss of PACs from the resin.
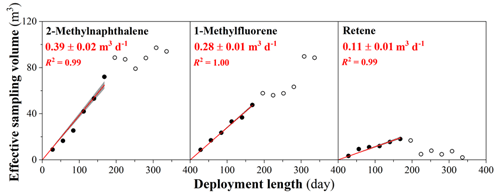
Figure 4The effective sampling volume of three representative PACs throughout the 48-week deployment period for XAD-PASs in Toronto. The markers indicate field-blank-corrected measured values, whereby data points marked with open symbols were excluded from the regression analysis. The red lines indicate linear regressions forced through the origin. The shaded areas represent the uncertainties of the calculated sampling rates obtained from the uncertainties of the slopes of these regressions. Sampling rates in m3 d−1 are obtained from the slope of these regressions.
Table 2Passive sampling rates (m3 d−1) of PACs determined in our study and reported in the literature.
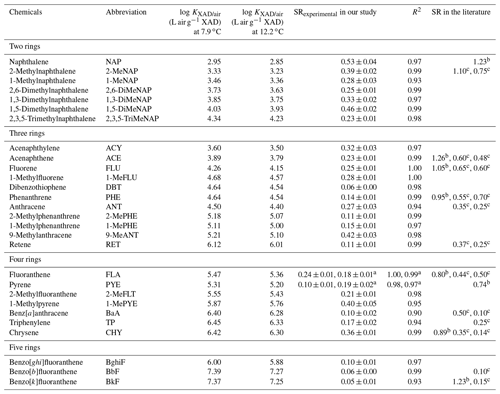
a Data for the clearing site from the Borden experiment. The originally calculated sampling rates are for the long-version XAD-PAS; i.e., the length of XAD mesh cylinders is 20 cm. Thus, in this table, they were converted to the sampling rates of short-version XAD-PAS (10 cm length) by dividing by 2 (Zhang et al., 2012), b Armitage et al. (2013), c Ellickson et al. (2017).
3.6 Is it possible to still derive useful kinetic information from the calibration experiments?
Veff values calculated using Eq. (1) are provided in Tables S7 and S9. Remarkably, when Veff for 15 PAHs, 12 alk-PAHs, and dibenzothiophene from the Toronto calibration experiment are plotted against deployment length, i.e., if we linearize the uptake curves from Figs. 1 and S2, almost all of the data show good linearity for the first 6 months of deployment (most linear regressions have R2>0.90 and p values <0.05, Table 2). Examples of such plots for 2-methylnaphthalene, 1-methylfluorene, and retene are shown in Fig. 4, with the remainder being compiled in Fig. S12. This is also true for fluoranthene and pyrene measured at the clearing site in Borden, but not at the forest site (Fig. S13). As only PUF plugs were used in the AASs to sample PAHs from the gas phase in Borden, five light PAHs (i.e., naphthalene, acenaphthylene, acenaphthene, fluorene, and phenanthrene) suffered from breakthrough, yielded unreliable Cair, and were therefore excluded from the data analysis.
As might be expected from the uptake curves, the Veff for most PACs levels off or decreases after 6 months (Figs. 3, S11, and S12). Nevertheless, the linear uptake within the first half-year of deployment suggests that the XAD-PAS can be used to sample PACs from the gas phase, even the most volatile one (naphthalene), as long as the deployment period does not exceed 6 months. Experimental sampling rates (SRexperimental) for 28 PACs, including 15 PAHs, 12 alk-PAHs, and dibenzothiophene, were estimated from the slopes of linear regressions for the data points from the first 6 months of deployment and ranged from 0.05 to 0.53 m3 d−1. Uncertainties of the SRexperimental values in the range of 2 % to 15 % were estimated from the standard error of the slopes of these linear regressions. For 14 compounds (2 PAHs, 11 alk-PAHs, and dibenzothiophene), these are the first SRs ever reported for the XAD-PAS. The SRexperimental values for fluoranthene and pyrene from the Toronto and Borden experiments are very close, lending support to the reliability of the two experiments. The actual sampling rates (SRactual) for 18 PACs were obtained using the simulated no-degradation uptake amounts in the first 6 months of deployment (Fig. S11) and AAS data in our Toronto experiment based on Eq. (1) (Table S11).
The SRexperimental values in this study are lower than those previously reported for PAHs (Armitage et al., 2013; Ellickson et al., 2017). The SRexperimental values for various SVOCs obtained from the same Toronto calibration experiment were also lower than those reported in other literature (Li et al., 2023a, b). The difference between the SRexperimental values calculated in our study and those previously reported may be caused by the difference in ambient wind speed (1.7, ∼4.0, and ∼8.0 m s−1 during our study, the study of Armitage et al., 2013, and the study of Ellickson et al., 2017, respectively) and possibly also in the rates of degradative loss. Other reasons for the relatively higher SRs in the study of Ellickson et al. (2017) may be due to (1) breakthrough losses for more volatile PAHs in a high-volume AAS, (2) failure to sample episodes of elevated air concentration when only sampling episodically with the AASs, and (3) lower air concentrations at active air sampling sites than those at passive air sampling sites.
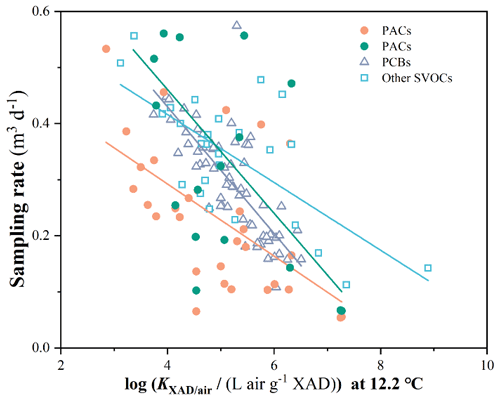
Figure 5The increase in the sampling rates of 120 chemicals with an increase in volatility. Different markers are used to indicate sampling rates of different chemical groups, and the lines in different colors indicate linear regressions between the sampling rate and log KXAD/air at 12.2 ∘C for different chemical groups. The dark green dots and line indicate the simulated sampling rates for PACs without degradation and linear regression for these PACs.
The reliability of SRexperimental and SRactual values for PACs presented here is supported by them falling within a similar range as those for other SVOCs from the same calibration experiment (Li et al., 2023a, b). Also, the SRs for PACs exhibit a consistent negative correlation with log KXAD/air, mirroring the pattern observed for other SVOCs (Fig. 5). However, the SRexperimental for PACs are relatively lower than those of the PCBs and other SVOCs with similar KXAD/air values, presumably due to the degradative loss, whereas the SRactual for PACs has a relationship with KXAD/air that is very similar to that of the PCBs (Fig. 5). A linear regression with the log (KXAD/air/(L air g−1 XAD)) at 12.2 ∘C yields the following relationship.
Going forward, we recommend that deployment periods for the XAD-PAS be kept shorter than 6 months if PACs are among the targeted SVOCs. We suggest that for such shorter deployments, the SRexperimental reported in Table 2 can be used. For PACs not included in Table 2, their SRexperimental and SRactual can be estimated using a predicted KXAD/air value and Eqs. (2) and (3). A quantitative interpretation of PAC levels in XAD-PAS deployed for periods longer than half a year may be compromised by the variable degradation loss of PACs from the XAD resin. This also applies retroactively to studies that have reported PAH levels in XAD-PAS deployed for 1 year; they should be considered to be semi-quantitative only (Daly et al., 2007; Lévy et al., 2018; Choi et al., 2009; Westgate et al., 2010; Abdul Hussain et al., 2019; Schummer et al., 2014; Schrlau et al., 2011; Shunthirasingham et al., 2011).
All data generated for this project are contained in the Supplement.
The supplement related to this article is available online at: https://doi.org/10.5194/amt-17-715-2024-supplement.
YL and FZ performed measurements and analyzed samples from the Toronto experiment. YS performed measurements and analyzed samples from the Borden experiment, both under the supervision of YDL. CS analyzed the atmospheric particle samples. YL performed the ozone flow tube experiment with guidance by ZZ and JPDA. YL and FZ performed the model simulations, and YL interpreted the data and wrote the paper under the guidance of FW. HH coordinated the project. All authors reviewed the paper.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
We are grateful to Rudy Boonstra for logistical help during fieldwork in Toronto and Bondi Gevao for help sampling in Borden.
This research has been supported by Environment and Climate Change Canada (grant no. GCXE20S008), the Canadian Foundation for Climate and Atmospheric Sciences, and a Connaught scholarship to Yuening Li.
This paper was edited by Pierre Herckes and reviewed by two anonymous referees.
Abdul Hussain, B., Westgate, J. N., Hayward, S. J., Shunthirasingham, C., Brown, T. N., Hung, H., Lei, Y. D., and Wania, F.: Polycyclic aromatic hydrocarbons and polychlorinated biphenyls in soils and atmosphere of Western Canadian mountains: The role of source proximity, precipitation, forest cover and mountain cold-trapping, Atmos. Environ. X, 1, 100004, https://doi.org/10.1016/j.aeaoa.2018.100004, 2019.
Ahad, J. M. E., Pakdel, H., Labarre, T., Cooke, C. A., Gammon, P. R., and Savard, M. M.: Isotopic analyses fingerprint sources of polycyclic aromatic compound-bearing dust in Athabasca oil sands region snowpack, Environ. Sci. Technol., 55, 5887–5897, https://doi.org/10.1021/acs.est.0c08339, 2021.
Andersson, J. T. and Achten, C.: Time to say goodbye to the 16 EPA PAHs? Toward an up-to-date use of PACs for environmental purposes, Polycycl. Aromat. Compd., 35, 330–354, https://doi.org/10.1080/10406638.2014.991042, 2015.
Armitage, J. M., Hayward, S. J., and Wania, F.: Modeling the uptake of neutral organic chemicals on XAD passive air samplers under variable temperatures, external wind speeds and ambient air concentrations (PAS-SIM), Environ. Sci. Technol., 47, 13546–13554, https://doi.org/10.1021/es402978a, 2013.
Atkinson, D. and Curthoys, G.: The determination of heats of adsorption by gas-solid chromatography, J. Chem. Educ., 55, 564–566, https://doi.org/10.1021/ed055p564, 1978.
Atkinson, R. and Arey, J.: Mechanisms of the gas-phase reactions of aromatic hydrocarbons and PAHs with OH and NO3 radicals, Polycycl. Aromat. Compd., 27, 15–40, https://doi.org/10.1080/10406630601134243, 2007.
ATSDR (Agency for Toxic Substances Disease Registry): Toxicological profile for polycyclic aromatic hydrocarbons, US Government Printing Office, https://wwwn.cdc.gov/TSP/ToxProfiles/ToxProfiles.aspx?id=122&tid=25 (last access: 1 July 2023), 1995.
Barthel, P., Thuens, S., Shunthirasingham, C., Westgate, J. N., Wania, F., and Radke, M.: Application of XAD-resin based passive air samplers to assess local (roadside) and regional patterns of persistent organic pollutants, Environ. Pollut., 166, 218–225, https://doi.org/10.1016/j.envpol.2012.03.026, 2012.
Bartkow, M. E., Hawker, D. W., Kennedy, K. E., and Müller, J. F.: Characterizing Uptake Kinetics of PAHs from the Air Using Polyethylene-Based Passive Air Samplers of Multiple Surface Area-to-Volume Ratios, Environ. Sci. Technol., 38, 2701–2706, https://doi.org/10.1021/es0348849, 2004.
Bedjanian, Y., Nguyen, M. L., and Le Bras, G.: Kinetics of the reactions of soot surface-bound polycyclic aromatic hydrocarbons with the OH radicals, Atmos. Environ., 44, 1754–1760, https://doi.org/10.1016/j.atmosenv.2010.02.007, 2010.
Berthiaume, A., Galarneau, E., and Marson, G.: Polycyclic aromatic compounds (PACs) in the Canadian environment: Sources and emissions, Environ. Pollut., 269, 116008, https://doi.org/10.1016/j.envpol.2020.116008, 2021.
Boffetta, P., Jourenkova, N., and Gustavsson, P.: Cancer risk from occupational and environmental exposure to polycyclic aromatic hydrocarbons, Cancer Causes Control, 8, 444–472, https://doi.org/10.1023/A:1018465507029, 1997.
Bohlin-Nizzetto, P., Melymuk, L., White, K. B., Kalina, J., Madadi, V. O., Adu-Kumi, S., Prokeš, R., Přibylová, P., and Klánová, J.: Field- and model-based calibration of polyurethane foam passive air samplers in different climate regions highlights differences in sampler uptake performance, Atmos. Environ., 238, https://doi.org/10.1016/j.atmosenv.2020.117742, 2020.
Bohlin, P., Audy, O., Škrdlíková, L., Kukučka, P., Vojta, Š., Přibylová, P., Prokeš, R., Čupr, P., and Klánová, J.: Evaluation and guidelines for using polyurethane foam (PUF) passive air samplers in double-dome chambers to assess semi-volatile organic compounds (SVOCs) in non-industrial indoor environments, Environ. Sci. Process. Impacts, 16, 2617–2626, https://doi.org/10.1039/c4em00305e, 2014a.
Bohlin, P., Audy, O., Škrdlíková, L., Kukučka, P., Přibylová, P., Prokeš, R., Vojta, Š., and Klánová, J.: Outdoor passive air monitoring of semi volatile organic compounds (SVOCs): a critical evaluation of performance and limitations of polyurethane foam (PUF) disks, Environ. Sci. Process. Impacts, 16, 433–444, https://doi.org/10.1039/C3EM00644A, 2014b.
Borrowman, C. K., Zhou, S., Burrow, T. E., and Abbatt, J. P. D.: Formation of environmentally persistent free radicals from the heterogeneous reaction of ozone and polycyclic aromatic compounds, Phys. Chem. Chem. Phys., 18, 205–212, https://doi.org/10.1039/c5cp05606c, 2016.
Boström, C. E., Gerde, P., Hanberg, A., Jernström, B., Johansson, C., Kyrklund, T., Rannug, A., Törnqvist, M., Victorin, K., and Westerholm, R.: Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air, Environ. Health Perspect., 110, 451–488, https://doi.org/10.1289/ehp.110-1241197, 2002.
Brubaker, W. W. and Hites, R. A.: OH Reaction Kinetics of Polycyclic Aromatic Hydrocarbons and Polychlorinated Dibenzo-p-dioxins and Dibenzofurans, J. Phys. Chem. A, 102, 915–921, https://doi.org/10.1021/jp9721199, 1998.
Chaemfa, C., Wild, E., Davison, B., Barber, J. L., and Jones, K. C.: A study of aerosol entrapment and the influence of wind speed, chamber design and foam density on polyurethane foam passive air samplers used for persistent organic pollutants, J. Environ. Monit., 11, 1135–1139, https://doi.org/10.1039/B823016A, 2009.
Cheng, H., Deng, Z., Chakraborty, P., Liu, D., Zhang, R., Xu, Y., Luo, C., Zhang, G., and Li, J.: A comparison study of atmospheric polycyclic aromatic hydrocarbons in three Indian cities using PUF disk passive air samplers, Atmos. Environ., 73, 16–21, https://doi.org/10.1016/j.atmosenv.2013.03.001, 2013.
Cheng, I., Wen, D., Zhang, L., Wu, Z., Qiu, X., Yang, F., and Harner, T.: Deposition mapping of polycyclic aromatic compounds in the oil sands region of Alberta, Canada and linkages to ecosystem impacts, Environ. Sci. Technol., 52, 12456–12464, https://doi.org/10.1021/acs.est.8b02486, 2018.
Choi, S.-D., Shunthirasingham, C., Daly, G. L., Xiao, H., Lei, Y. D., and Wania, F.: Levels of polycyclic aromatic hydrocarbons in Canadian mountain air and soil are controlled by proximity to roads, Environ. Pollut., 157, 3199–3206, https://doi.org/10.1016/j.envpol.2009.05.032, 2009.
Daly, G. L., Lei, Y. D., Castillo, L. E., Muir, D. C. G., and Wania, F.: Polycyclic aromatic hydrocarbons in Costa Rican air and soil: A tropical/temperate comparison, Atmos. Environ., 41, 7339–7350, https://doi.org/10.1016/j.atmosenv.2007.05.014, 2007.
Domínguez-Morueco, N., Augusto, S., Trabalón, L., Pocurull, E., Borrull, F., Schuhmacher, M., Domingo, J. L., and Nadal, M.: Monitoring PAHs in the petrochemical area of Tarragona County, Spain: comparing passive air samplers with lichen transplants, Environ. Sci. Pollut. Res., 24, 11890–11900, https://doi.org/10.1007/s11356-015-5612-2, 2017.
Ellickson, K. M., McMahon, C. M., Herbrandson, C., Krause, M. J., Schmitt, C. M., Lippert, C. J., and Pratt, G. C.: Analysis of polycyclic aromatic hydrocarbons (PAHs) in air using passive sampling calibrated with active measurements, Environ. Pollut., 231, 487–496, https://doi.org/10.1016/j.envpol.2017.08.049, 2017.
Environment Canada and Health Canada: Canadian Environmental Protection Act Polycyclic Aromatic Hydrocarbons-Priority Substances List Assessment Report, Ottawa, Ontario, 1–68, ISBN 0-662-22209-1, 1994.
Esteve, W., Budzinski, H., and Villenave, E.: Relative rate constants for the heterogeneous reactions of OH, NO2 and NO radicals with polycyclic aromatic hydrocarbons adsorbed on carbonaceous particles, Part 1: PAHs adsorbed on 1–2 µm calibrated graphite particles, Atmos. Environ., 38, 6063–6072, https://doi.org/10.1016/j.atmosenv.2004.05.059, 2004.
Esteve, W., Budzinski, H., and Villenave, E.: Relative rate constants for the heterogeneous reactions of NO2 and OH radicals with polycyclic aromatic hydrocarbons adsorbed on carbonaceous particles. Part 2: PAHs adsorbed on diesel particulate exhaust SRM 1650a, Atmos. Environ., 40, 201–211, https://doi.org/10.1016/j.atmosenv.2005.07.053, 2006.
Ghosal, D., Ghosh, S., Dutta, T. K., and Ahn, Y.: Current state of knowledge in microbial degradation of polycyclic aromatic hydrocarbons (PAHs): A review, Front. Microbiol., 7, 1369, https://doi.org/10.3389/fmicb.2016.01369, 2016.
Golzadeh, N., Barst, B. D., Baker, J. M., Auger, J. C., and McKinney, M. A.: Alkylated polycyclic aromatic hydrocarbons are the largest contributor to polycyclic aromatic compound concentrations in traditional foods of the Bigstone Cree Nation in Alberta, Canada, Environ. Pollut., 275, 116625, https://doi.org/10.1016/j.envpol.2021.116625, 2021.
Goss, K. U.: Adsorption of VOCs from the gas phase to different minerals and a mineral mixture, Environ. Sci. Technol., 30, 2135–2142, https://doi.org/10.1021/es950508f, 1996.
Grosjean, D., Fung, K., and Harrison, J.: Interactions of polycyclic aromatic hydrocarbons with atmospheric pollutants, Environ. Sci. Technol., 17, 673–679, https://doi.org/10.1021/es00117a010, 1983.
Grung, M., Næs, K., Fogelberg, O., Nilsen, A. J., Brack, W., Lübcke-von Varel, U., and Thomas, K. V: Effects-directed analysis of sediments from polluted marine sites in Norway, J. Toxicol. Env. Health, 74, 439–454, https://doi.org/10.1080/15287394.2011.550555, 2011.
Harner, T., Su, K., Genualdi, S., Karpowicz, J., Ahrens, L., Mihele, C., Schuster, J., Charland, J.-P. P., and Narayan, J.: Calibration and application of PUF disk passive air samplers for tracking polycyclic aromatic compounds (PACs), Atmos. Environ., 75, 123–128, https://doi.org/10.1016/j.atmosenv.2013.04.012, 2013.
Harner, T., Rauert, C., Muir, D., Schuster, J. K., Hsu, Y.-M., Zhang, L., Marson, G., Watson, J. G., Ahad, J., and Cho, S.: Air synthesis review: polycyclic aromatic compounds in the oil sands region, Environ. Rev., 26, 430–468, https://doi.org/10.1139/er-2018-0039, 2018.
Hawthorne, S. B., Miller, D. J., and Kreitinger, J. P.: Measurement of total polycyclic aromatic hydrocarbon concentrations in sediments and toxic units used for estimating risk to benthic invertebrates at manufactured gas plant sites, Environ. Toxicol. Chem., 25, 287–296, https://doi.org/10.1897/05-111R.1, 2006.
Hayward, S. J., Lei, Y. D., and Wania, F.: Sorption of a diverse set of organic chemical vapors onto XAD-2 resin: Measurement, prediction and implications for air sampling, Atmos. Environ., 45, 296–302, https://doi.org/10.1016/j.atmosenv.2010.10.028, 2011.
Holme, J. A., Valen, H., Brinchmann, B. C., Vist, G. E., Grimsrud, T. K., Becher, R., Holme, A. M., Øvrevik, J., and Alexander, J.: Polycyclic aromatic hydrocarbons (PAHs) may explain the paradoxical effects of cigarette use on preeclampsia (PE), Toxicology, 473, 153206, https://doi.org/10.1016/j.tox.2022.153206, 2022.
Holt, E., Bohlin-Nizzetto, P., Borůvková, J., Harner, T., Kalina, J., Melymuk, L., and Klánová, J.: Using long-term air monitoring of semi-volatile organic compounds to evaluate the uncertainty in polyurethane-disk passive sampler-derived air concentrations, Environ. Pollut., 220, 1100–1111, https://doi.org/10.1016/j.envpol.2016.11.030, 2017.
Jariyasopit, N., Liu, Y., Liggio, J., and Harner, T.: Stability of polycyclic aromatic compounds in polyurethane foam-type passive air samplers upon O3 exposure, Atmos. Environ., 120, 200–204, https://doi.org/10.1016/j.atmosenv.2015.08.088, 2015.
Jariyasopit, N., Zhang, Y., Martin, J. W., and Harner, T.: Comparison of polycyclic aromatic compounds in air measured by conventional passive air samplers and passive dry deposition samplers and contributions from petcoke and oil sands ore, Atmos. Chem. Phys., 18, 9161–9171, https://doi.org/10.5194/acp-18-9161-2018, 2018.
Jariyasopit, N., Tung, P., Su, K., Halappanavar, S., Evans, G. J., Su, Y., Khoomrung, S., and Harner, T.: Polycyclic aromatic compounds in urban air and associated inhalation cancer risks: A case study targeting distinct source sectors, Environ. Pollut., 252, 1882–1891, https://doi.org/10.1016/j.envpol.2019.06.015, 2019.
Jariyasopit, N., Harner, T., Shin, C., and Park, R.: The effects of plume episodes on PAC profiles in the Athabasca oil sands region, Environ. Pollut., 282, 117014, https://doi.org/10.1016/j.envpol.2021.117014, 2021.
Kaisarevic, S., Varel, U. L., Orcic, D., Streck, G., Schulze, T., Pogrmic, K., Teodorovic, I., Brack, W., and Kovacevic, R.: Effect-directed analysis of contaminated sediment from the wastewater canal in Pancevo industrial area, Serbia, Chemosphere, 77, 907–913, https://doi.org/10.1016/j.chemosphere.2009.08.042, 2009.
Kasumba, J. and Holmén, B. A.: Heterogeneous ozonation reactions of PAHs and fatty acid methyl esters in biodiesel particulate matter, Atmos. Environ., 175, 15–24, https://doi.org/10.1016/j.atmosenv.2017.11.051, 2018.
Kim, K. H., Jahan, S. A., Kabir, E., and Brown, R. J. C.: A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects, Environ. Int., 60, 71–80, https://doi.org/10.1016/j.envint.2013.07.019, 2013.
Lévy, M., Al-Alam, J., Ridacker, C., Massemin, S., and Millet, M.: Use of XAD®-2 passive air samplers for monitoring environmental trends of PAHs, PCBs and pesticides in three different sites in Strasbourg and its vicinity (east of France), Atmos. Environ., 195, 12–23, https://doi.org/10.1016/j.atmosenv.2018.09.052, 2018.
Lewis, R. G. and Coutant, R. W.: Determination of phase-distributed polycyclic aromatic hydrocarbons in air by grease-coated denuders, in: Gas and Particle Phase Measurements of Atmospheric Organic Compounds, CRC Press, 201–231, ISBN 90-5699-647-9, 2020.
Li, Y. and Wania, F.: Partitioning between polyurethane foam and the gas phase: Data compilation, uncertainty estimation and implications for air sampling, Environ. Sci. Process. Impacts, 23, 723–734, https://doi.org/10.1039/d1em00036e, 2021.
Li, Y., Armitage, J. M., and Wania, F.: Graphical tools for the planning and interpretation of polyurethane foam based passive air sampling campaigns, Environ. Sci. Process. Impacts, 24, 414–425, https://doi.org/10.1039/D1EM00559F, 2022.
Li, Y., Zhan, F., Lei, Y. D., Shunthirasingham, C., Hung, H., and Wania, F.: Field calibration and PAS-SIM model evaluation of the XAD-based passive air samplers for semi-volatile organic compounds, Environ. Sci. Technol., 57, 9224–9233, https://doi.org/10.1021/acs.est.3c00809, 2023a.
Li, Y., Zhan, F., Shunthirasingham, C., Lei, Y. D., Hung, H., and Wania, F.: Unbiased passive sampling of all polychlorinated biphenyls congeners from air, Environ. Sci. Technol. Lett., 10, 565–572, https://doi.org/10.1021/acs.estlett.3c00271, 2023b.
Lima, A. L. C., Farrington, J. W., and Reddy, C. M.: Combustion-derived polycyclic aromatic hydrocarbons in the environment-a review, Environ. Forensics, 6, 109–131, https://doi.org/10.1080/15275920590952739, 2005.
Liu, Y. N., Tao, S., Dou, H., Zhang, T. W., Zhang, X. L., and Dawson, R.: Exposure of traffic police to Polycyclic aromatic hydrocarbons in Beijing, China, Chemosphere, 66, 1922–1928, https://doi.org/10.1016/j.chemosphere.2006.07.076, 2007.
Mahoney, C., Montgomery, J., Connor, S., and Cobbaert, D.: Oil sands wetland ecosystem monitoring program indicators in Alberta, Canada: Transitioning from pilot to long-term monitoring, Water, 15, 1914, https://doi.org/10.3390/w15101914, 2023.
Masclet, P., Hoyau, V., Jaffrezo, J. L., and Cachier, H.: Polycyclic aromatic hydrocarbon deposition on the ice sheet of Greenland. Part I: superficial snow, Atmos. Environ., 34, 3195–3207, https://doi.org/10.1016/S1352-2310(99)00196-X, 2000.
Meierdierks, J., Zarfl, C., Beckingham, B., and Grathwohl, P.: Unique calibration of passive air sampling for field monitoring of PAHs with polyethylene thin films across seasons and locations, Environ. Sci. Atmos., 1, 253–266, https://doi.org/10.1039/D0EA00022A, 2021.
Melymuk, L., Robson, M., Helm, P. A., and Diamond, M. L.: Evaluation of passive air sampler calibrations: Selection of sampling rates and implications for the measurement of persistent organic pollutants in air, Atmos. Environ., 45, 1867–1875, https://doi.org/10.1016/j.atmosenv.2011.01.011, 2011.
Melymuk, L., Bohlin-Nizzetto, P., Prokeš, R., Kukučka, P., Přibylová, P., Vojta, Š., Kohoutek, J., Lammel, G., and Klánová, J.: Uncertainties in monitoring of SVOCs in air caused by within-sampler degradation during active and passive air sampling, Atmos. Environ., 167, 553–565, https://doi.org/10.1016/j.atmosenv.2017.08.038, 2017.
Moradi, M., Hung, H., Li, J., Park, R., Shin, C., Alexandrou, N., Iqbal, M. A., Takhar, M., Chan, A., and Brook, J. R.: Assessment of alkylated and unsubstituted polycyclic aromatic hydrocarbons in air in urban and semi-urban areas in Toronto, Canada, Environ. Sci. Technol., 56, 2959–2967, https://doi.org/10.1021/acs.est.1c04299, 2022.
Moradi, M., Eng, A., Staebler, R., and Harner, T.: Atmospheric emissions estimation of polycyclic aromatic compounds from an oil sands tailings pond using passive air samplers, Chemosphere, 345, 140423, https://doi.org/10.1016/j.chemosphere.2023.140423, 2023.
Muir, D. C. G. and Galarneau, E.: Polycyclic aromatic compounds (PACs) in the Canadian environment: Links to global change, Environ. Pollut., 273, 116425, https://doi.org/10.1016/j.envpol.2021.116425, 2021.
Nikolaou, K., Masclet, P., and Mouvier, G.: Sources and chemical reactivity of polynuclear aromatic hydrocarbons in the atmosphere — A critical review, Sci. Total Environ., 32, 103–132, https://doi.org/10.1016/0048-9697(84)90125-6, 1984.
Nolan, R. H., Anderson, L. O., Poulter, B., and Varner, J. M.: Increasing threat of wildfires: the year 2020 in perspective: A Global Ecology and Biogeography special issue, Glob. Ecol. Biogeogr., 31, 1898–1905, https://doi.org/10.1111/geb.13588, 2022.
Pcchillips, D. H., Grover, P. L., and Sims, P.: A quantitative determination of the covalent binding of a series of polycylic hydrocarbons to dna in mouse skin, Int. J. Cancer, 23, 201–208, https://doi.org/10.1002/ijc.2910230211, 1979.
Pitts, J. N., Lokensgard, D. M., Ripley, P. S., Van Cauwenberghe, K. A., Van Vaeck, L., Shaffer, S. D., Thill, A. J., and Belser, W. L.: “Atmospheric” Epoxidation of benzo[a]pyrene by ozone: Formation of the metabolite benzo[a]pyrene-4,5-oxide, Science, 210, 1347–1349, https://doi.org/10.1126/science.210.4476.1347, 1980.
Pozo, K., Estellano, V. H., Harner, T., Diaz-Robles, L., Cereceda-Balic, F., Etcharren, P., Pozo, K., Vidal, V., Guerrero, F., and Vergara-Fernández, A.: Assessing polycyclic aromatic hydrocarbons (PAHs) using passive air sampling in the atmosphere of one of the most wood-smoke-polluted cities in Chile: The case study of Temuco, Chemosphere, 134, 475–481, https://doi.org/10.1016/j.chemosphere.2015.04.077, 2015.
United Nations Environment Programme: Polycyclic Aromatic Hydrocarbons (PAHs) – Assessment Report on Issues of Concern, 1–2, https://wedocs.unep.org/20.500.11822/41460 (last access: 20 September 2023), 2020.
Rauert, C., Harner, T., Ahad, J. M. E., and Percy, K. E.: Using tree cores to evaluate historic atmospheric concentrations and trends of polycyclic aromatic compounds in the Oil Sands region of Alberta, Canada, Sci. Total Environ., 739, 139996, https://doi.org/10.1016/j.scitotenv.2020.139996, 2020.
Ravindra, K., Wauters, E., and Van Grieken, R.: Variation in particulate PAHs levels and their relation with the transboundary movement of the air masses, Sci. Total Environ., 396, 100–110, https://doi.org/10.1016/j.scitotenv.2008.02.018, 2008.
Sarma, S. N., Blais, J. M., and Chan, H. M.: Neurotoxicity of alkylated polycyclic aromatic compounds in human neuroblastoma cells, J. Toxicol. Env. Health, 80, 285–300, https://doi.org/10.1080/15287394.2017.1314840, 2017.
Schrlau, J. E., Geiser, L., Hageman, K. J., Landers, D. H., and Simonich, S. M.: Comparison of lichen, conifer needles, passive air sampling devices, and snowpack as passive sampling media to measure semi-volatile organic compounds in remote atmospheres, Environ. Sci. Technol., 45, 10354–10361, https://doi.org/10.1021/es202418f, 2011.
Schummer, C., Appenzeller, B. M., and Millet, M.: Monitoring of polycyclic aromatic hydrocarbons (PAHs) in the atmosphere of southern Luxembourg using XAD-2 resin-based passive samplers, Environ. Sci. Pollut. Res., 21, 2098–2107, https://doi.org/10.1007/s11356-013-2106-y, 2014.
Shen, G., Tao, S., Wei, S., Zhang, Y., Wang, R., Wang, B., Li, W., Shen, H., Huang, Y., Yang, Y., Wang, W., Wang, X., and Simonich, S. L. M.: Retene emission from residential solid fuels in China and evaluation of retene as a unique marker for soft wood combustion, Environ. Sci. Technol., 46, 4666–4672, https://doi.org/10.1021/es300144m, 2012.
Shunthirasingham, C., Barra, R., Mendoza, G., Montory, M., Oyiliagu, C. E., Lei, Y. D., and Wania, F.: Spatial variability of atmospheric semivolatile organic compounds in Chile, Atmos. Environ., 45, 303–309, https://doi.org/10.1016/j.atmosenv.2010.10.027, 2011.
Su, Y., Lei, Y. D., Wania, F., Shoeib, M., and Harner, T.: Regressing gas/particle partitioning data for polycyclic aromatic hydrocarbons, Environ. Sci. Technol., 40, 3558–3564, https://doi.org/10.1021/es052496w, 2006.
Su, Y., Wania, F., Harner, T., and Lei, Y. D.: Deposition of polybrominated diphenyl ethers, polychlorinated biphenyls, and polycyclic aromatic hydrocarbons to a boreal deciduous forest, Environ. Sci. Technol., 41, 534–540, https://doi.org/10.1021/es0622047, 2007a.
Su, Y., Wania, F., Ying, D. L., Harner, T., and Shoeib, M.: Temperature dependence of the air concentrations of polychlorinated biphenyls and polybrominated diphenyl ethers in a forest and a clearing, Environ. Sci. Technol., 41, 4655–4661, https://doi.org/10.1021/es070334p, 2007b.
Terzi, E. and Samara, C.: Gas-particle partitioning of polycyclic aromatic hydrocarbons in urban, adjacent coastal, and continental background sites of western Greece, Environ. Sci. Technol., 38, 4973–4978, https://doi.org/10.1021/es040042d, 2004.
Tokiwa, H., Nakagawa, R., Morita, K., and Ohnishi, Y.: Mutagenicity of nitro derivatives induced by exposure of aromatic compounds to nitrogen dioxide, Mutat. Res. Mutagen. Relat. Subj., 85, 195–205, https://doi.org/10.1016/0165-1161(81)90036-4, 1981.
Tromp, P. C., Beeltje, H., Okeme, J. O., Vermeulen, R., Pronk, A., and Diamond, M. L.: Calibration of polydimethylsiloxane and polyurethane foam passive air samplers for measuring semi volatile organic compounds using a novel exposure chamber design, Chemosphere, 227, 435–443, https://doi.org/10.1016/j.chemosphere.2019.04.043, 2019.
UFZ-LSER: database v 3.2.1 [Internet] http://www.ufz.de/lserd, last access: 13 July 2022.
Van Vaeck, L. and Van Cauwenberghe, K.: Conversion of polycyclic aromatic hydrocarbons on diesel particulate matter upon exposure to ppm levels of ozone, Atmos. Environ., 18, 323–328, https://doi.org/10.1016/0004-6981(84)90106-9, 1984.
Wakeham, S. G., Schaffner, C., and Giger, W.: Poly cyclic aromatic hydrocarbons in recent lake sediments – II. Compounds derived from biogenic precursors during early diagenesis, Geochim. Cosmochim. Ac., 44, 415–429, 1980.
Wania, F. and Shunthirasingham, C.: Passive air sampling for semi-volatile organic chemicals, Environ. Sci. Process. Impacts, 22, 1925–2002, https://doi.org/10.1039/d0em00194e, 2020.
Wania, F., Shen, L., Lei, Y. D., Teixeira, C., and Muir, D. C. G.: Development and calibration of a resin-based passive sampling system for monitoring persistent organic pollutants in the atmosphere, Environ. Sci. Technol., 37, 1352–1359, https://doi.org/10.1021/es026166c, 2003.
Westgate, J. N., Shunthirasingham, C., Oyiliagu, C. E., von Waldow, H., and Wania, F.: Three methods for quantifying proximity of air sampling sites to spatially resolved emissions of semi-volatile organic contaminants, Atmos. Environ., 44, 4380–4387, https://doi.org/10.1016/j.atmosenv.2010.07.051, 2010.
White, P. A.: The genotoxicity of priority polycyclic aromatic hydrocarbons in complex mixtures, Mutat. Res. Toxicol. Environ. Mutagen., 515, 85–98, https://doi.org/10.1016/S1383-5718(02)00017-7, 2002.
Wnorowski, A., Aklilu, Y., Harner, T., Schuster, J., and Charland, J.-P.: Polycyclic aromatic compounds in ambient air in the surface minable area of Athabasca oil sands in Alberta (Canada), Atmos. Environ., 244, 117897, https://doi.org/10.1016/j.atmosenv.2020.117897, 2021.
Wnorowski, A., Harnish, D., Jiang, Y., Celo, V., Dabek-Zlotorzynska, E., and Charland, J. P.: Assessment and characterization of alkylated pahs in selected sites across Canada, Atmosphere, 13, 1–16, https://doi.org/10.3390/atmos13081320, 2022.
World Health Organization: Selected Non-Heterocyclic Polycyclic Aromatic Hydrocarbons – Environmental Health Criteria 202, World Health Organization, Geneva, 1–899, ISBN 92-4-1572027, 1998.
Xu, S., Liu, W., and Tao, S.: Emission of polycyclic aromatic hydrocarbons in China, Environ. Sci. Technol., 40, 702–708, https://doi.org/10.1021/es0517062, 2006.
Zhang, X. and Wania, F.: Modeling the uptake of semivolatile organic compounds by passive air samplers: importance of mass transfer processes within the porous sampling media, Environ. Sci. Technol., 46, 9563–9570, https://doi.org/10.1021/es302334r, 2012.
Zhang, X., Tsurukawa, M., Nakano, T., Lei, Y. D., and Wania, F.: Sampling medium side resistance to uptake of semivolatile organic compounds in passive air samplers, Environ. Sci. Technol., 45, 10509–10515, https://doi.org/10.1021/es2032373, 2011.
Zhang, X., Wong, C., Lei, Y. D., and Wania, F.: Influence of sampler configuration on the uptake kinetics of a passive air sampler, Environ. Sci. Technol., 45, 397–403, https://doi.org/10.1021/es203292x, 2012.
Zhou, S., Hwang, B. C. H., Lakey, P. S. J., Zuend, A., Abbatt, J. P. D., and Shiraiwa, M.: Multiphase reactivity of polycyclic aromatic hydrocarbons is driven by phase separation and diffusion limitations, P. Natl. Acad. Sci. USA, 116, 11658–11663, https://doi.org/10.1073/pnas.1902517116, 2019.
Zhou, Z. and Abbatt, J. P. D.: Formation of gas-phase hydrogen peroxide via multiphase ozonolysis of unsaturated lipids, Environ. Sci. Technol. Lett., 8, 114–120, https://doi.org/10.1021/acs.estlett.0c00757, 2021.