the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Enhancing forest air sampling using a novel reusable ozone filter design
Thomas Mayer
Helko Borsdorf
Biogenic volatile organic compounds (BVOCs), such as monoterpenes, play essential roles in ecological and atmospheric processes, influencing air quality, climate, and interspecies interactions. For accurate identification and quantification of these reactive compounds in the environment, active sampling on sorbent tubes followed by thermal desorption–gas chromatography–mass spectrometry is commonly used. However, ozone present in the sampled air can degrade both the analytes and the sorbent material during the sampling process, leading to underestimation of target substances and overestimation of their degradation products. This study evaluates a novel reusable ozone filter designed for direct attachment to sorbent tubes and compatibility with multi-tube samplers. The filter utilizes potassium iodide (KI) or sodium thiosulfate (Na2S2O3) deposited on reusable glass filters and copper wool to improve the accuracy of BVOC measurements. Both types of ozone scrubbers were tested under varying ozone concentrations up to 50 ppb and relative humidity levels up to 90 %, utilizing a straightforward load-and-flush method as well as a permeation approach that simulates field sampling conditions. Furthermore, both methods were compared regarding their suitability for the systematic evaluation of ozone filters.
Results indicate that both KI and Na2S2O3 effectively remove ozone, with KI showing a slightly higher performance and lower dependence on relative humidity, maintaining over 90 % removal efficiency even after 10 d of ambient air exposure. Recovery rates for four structurally different monoterpenes (α-pinene, myrcene, limonene, linalool) showed no significant differences between filtered and unfiltered samples at baseline ozone concentrations, demonstrating that the ozone filters did not negatively impact analyte recovery. When no filter was used, recovery rates for myrcene, limonene, and linalool declined with increasing ozone concentration while showing a method-dependent positive influence of increasing relative humidity. Both scrubber materials maintained high and comparable recovery rates across all tested conditions, except at very low relative humidity, thereby enhancing measurement accuracy and comparability under diverse environmental scenarios. Field tests confirmed the effectiveness of KI-loaded scrubbers in enhancing monoterpene detection in forest air while safeguarding the sorbent material. These results, combined with the easy reusability of the glass filters and the absence of additional equipment or power requirements, highlight that this scrubber design proves to be an optimal choice for the long-term environmental monitoring of volatile organic compounds.
- Article
(2670 KB) - Full-text XML
-
Supplement
(912 KB) - BibTeX
- EndNote
Biogenic volatile organic compounds (BVOCs) are a diverse group of organic substances emitted into the atmosphere by a wide range of organisms, with plants representing a significant source (Guenther et al., 1995, 2012; Sindelarova et al., 2014). These compounds, which include isoprene, monoterpenes, and sesquiterpenes, play a crucial role in ecological and atmospheric processes. BVOCs significantly influence the chemical composition of the atmosphere by contributing to the formation of ozone and secondary organic aerosols (SOAs), affecting both climate and air quality (Hallquist et al., 2009). They are also involved in communication and interaction mechanisms between organisms within a species or across species boundaries and in plants' defense mechanisms against biotic and abiotic stress, underlining their ecological importance (Laothawornkitkul et al., 2009).
In order to investigate and understand both the atmospheric and ecological processes, it is essential to conduct a comprehensive analysis of concentrations and the composition of these compounds in the environment. A common method for obtaining both qualitative and quantitative information on BVOCs in air samples combines active or passive sampling with thermal desorption–gas chromatography–mass spectrometry (TD–GC–MS). In this approach, BVOCs present in the air are enriched on a suitable sorbent material over a sampling period ranging from minutes to hours. Subsequently, the enriched compounds are thermally desorbed and separated based on their respective physico-chemical properties, followed by identification and quantification based on their compound-specific mass-to-charge ratios. By enriching large volumes of air, this method offers high sensitivity down to concentrations in the µg m−3 range and high specificity, enabling the detection and differentiation of compounds, including isomers (Borsdorf et al., 2023; Hellén et al., 2024). Although this approach has certain benefits, it is not without its shortcomings. Aside from the time-consuming processes of sample collection, transportation to the laboratory, analysis, and data evaluation, the accuracy of the measurements can be affected by environmental conditions during sampling, such as humidity and ozone.
While carbon-based sorbents are negatively affected by humidity levels above 40 % relative humidity (rH), hydrophobic polymer-based sorbents like Tenax TA work well over a wide relative humidity range (Maceira et al., 2017). Additionally, ozone can degrade the sorbent material because of its high reactivity, leading to the formation of artifacts and breakdown products (Hwan Lee et al., 2006), which may complicate the identification and quantification of target substances by co-elution and enhanced background noise. Furthermore, the adsorbents' efficiency may be negatively affected, leading to underestimation of the BVOC concentrations and lower recovery rates. Besides the reaction with the sorbent material, adsorbed BVOCs with one or more C–C double bond may be decomposed by ozonation, leading to loss of analytes and possible detection of transformation products (Calogirou et al., 1996). Hence, the removal of ozone before it gets in contact with the sorbent or adsorbed BVOCs is a crucial step to enhance the measurements' accuracy. To date, various ozone scrubber materials and methods have been used, such as heated stainless-steel tubes (Hellén et al., 2012), NO titration (Pollmann et al., 2005), manganese oxide (Fick et al., 2001), sodium thiosulfate (Ernle et al., 2023), or potassium iodide/copper (Borsdorf et al., 2023; Hellén et al., 2024). Although the different ozone scrubbers are able to effectively remove ozone, they have their specific disadvantages, such as loss of some analytes (NO titration, manganese oxide), safety concerns (NO titration), the need for additional devices and power supply (NO titration, heated stainless-steel tubes), limited compatibility with multi-tube samplers, and the necessity of regular checks and replacement during measurement campaigns (Hellén et al., 2024; Pollmann et al., 2005).
Various approaches have been employed to assess the influence of ozone and humidity on analyte recovery or to evaluate the efficiency of ozone filters. Methods that simulate realistic environmental concentrations include the diffusion of gas-phase analyte molecules through a glass tube (Calogirou et al., 1996; Helmig et al., 2003; Pollmann et al., 2005) or the introduction of diluted gas standard mixtures (Ernle et al., 2023; Mermet et al., 2019), both analogous to the permeation approach used in this study. In contrast, approaches involving the direct addition of analytes or methanolic solutions (Fick et al., 2001; Hellén et al., 2024) may introduce uncertainties due to artificially high analyte concentrations or the presence of solvent molecules. Furthermore, loading standard mixtures onto sorbent tubes and subsequently flushing them with ozone-containing humid air enables the investigation of post-adsorption analyte degradation during sampling. To the best of our knowledge, no study has yet been conducted that has systematically evaluated the influence of ozone filters on both the entire sampling process and post-adsorption degradation processes depending on ozone concentration and relative humidity.
For the application in environmental monitoring using multi-tube samplers, ozone scrubbers must be compact, cost-effective, easy to use, stable against various and changing environmental conditions, and highly efficient in preventing analyte degradation while maintaining the integrity of collected samples. In this study, we developed, optimized, and validated an ozone filter tailored for such sampling setups that avoids the disadvantages of previous ozone filter approaches. Our ozone scrubber can be directly placed before the inlet of a common sorbent tube and equipped with different ozone-depleting reagents (KI or Na2S2O3). Our primary objective was to assess the ozone removal efficiency of these scrubbers and their suitability for long-term environmental sampling of terpenes in forest air. To achieve this, we compared the breakthrough of ozone under realistic environmental conditions for typical sampling durations and assessed the long-term stability of the filters. Furthermore, we systematically evaluated the influence of both scrubber materials on analyte recovery under different environmental conditions using two complementary methods: a load-and-flush approach, where analytes are preloaded onto sorbent tubes and subsequently exposed to ozone-containing humid air, and a permeation approach, which more closely simulates a real-world sampling scenario by enriching analytes from an ozone- and humidity-controlled airstream. Additionally, we aim to determine the most suitable validation approach for ozone scrubbers to provide a reliable assessment of the ozone scrubber performance in field applications. Finally, the proposed ozone scrubber setup was tested under real environmental conditions.
2.1 Chemicals and materials
Methanol (SupraSolv for GC–MS, Merck KGaA, Germany) was used for preparing standards and cleaning ozone scrubbers. Ultrapure, particle-free Milli-Q water (Milli-Q Direct 8, Merck KGaA, Germany) was used to clean the sintered glass filters. Ozone scrubbers were loaded with potassium iodide (KI; >99.0 %, Th. Geyer GmbH & Co. KG, Germany) or sodium thiosulfate (Na2S2O3, >99.5 %, pentahydrate, Riedel-deHaën, Germany). Zero air was generated using a laboratory compressor (SICOLAB 062, Dürr Technik GmbH & Co. KG, Germany). The relative humidity of airstreams was adjusted by passing through ultrapure water (LiChrosolv for chromatography, Merck KGaA, Germany). Copper wool (∼ 99 %, Carl Roth GmbH & Co. KG, Germany) was placed between the tube caps and ozone scrubbers to prevent reactive iodine produced during ozone removal with KI from entering the sorption tubes. “Bio-monitoring” sorbent tubes (Markes International Ltd., UK) were used for all experiments and pre-conditioned according to the manufacturer's instructions using a TC-20 tube conditioner (Markes International Ltd., UK). Hydrogen carrier gas was produced in-lab using a HyGen200 hydrogen generator (Claind srl, Italy). Myrcen, limonene, α-pinene (all Sigma Aldrich, USA), and linalool (Glentham Life Sciences Ltd., UK) were used as reference substances. Terpenes MegaMix Standard #1 (Restek GmbH, Germany) was used as a calibration standard and bromobenzene-d5 (DeuChem GmbH, Germany) as an internal standard.
2.2 Ozone scrubber design
The small ozone scrubber has been designed to be compatible with commonly used adsorption tubes and diffusion-locking caps (Fig. 1), making it compatible with multi-tube samplers. A glass filter, loaded with the ozone-depleting reagent, is encased in a stainless-steel housing with a screw cap, thereby facilitating rapid and straightforward replacement of the filter. Two O-rings, located on top of the glass filter and between the housing and the diffusion-locking cap, prevent the sampled air from bypassing the filter. Furthermore, copper wool, positioned beneath the glass filter, inhibits the entry of reactive iodine species into the adsorption tube when KI is used as the scrubber material and serves as an additional reaction site for ozone depletion. By easily replacing the glass filter and the copper wool, the ozone scrubber can be prepared and reused for the next sampling within a few minutes. The reusable glass filters can easily be cleaned by rinsing with water and methanol.
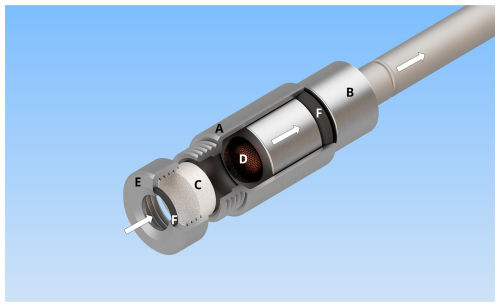
Figure 1Schematic layout of the ozone scrubber. The scrubber housing (a) was designed to fit on a commonly used diffusion-locking cap (b). Sample airflows from the left to the right, passing both a loaded glass filter as an ozone scrubber (c) and copper wool (d). A screw cap (e) facilitates the quick and easy exchange of the filter. Two O-rings (f), located between the screw cap and the filter and between the housing and the diffusion-locking cap, prevent sampled air from bypassing the filter. Arrows indicate the direction of the airflow through the ozone filter.
2.3 Preparation of ozone filters
Ozone scrubbers were prepared by loading cleaned and dried glass filters (VitraPOR, 9 mm diameter, 3.5 mm height, 100–160 µm pore diameter, ROBU Glasfilter-Geräte GmbH, Germany) with freshly prepared solutions of KI or Na2S2O3. The solutions were prepared by weighing 40 g of the respective salt and subsequent dissolution with 360 g of Milli-Q water, resulting in a concentration of 10 % . For loading the ozone filters, the glass filters were placed in a specially designed, 3D-printed cleaning device made of cyclic olefin copolymer (IWK Institut für Werkstofftechnik und Kunststoffverarbeitung, Switzerland) and rinsed with the solution until completely wetted. Afterwards, the filters were placed on filter paper to remove excess solution and dried overnight at room temperature. After use, the filters were soaked in ultrapure Milli-Q water, flushed with 100 mL of Milli-Q water each using the 3D-printed cleaning device, rinsed with methanol in an ultrasonic bath, and dried overnight.
2.4 Characterization of ozone filters
2.4.1 Deposition of KI and Na2S2O3 on glass filters
To quantify the amount of the ozone-depleting reagent deposited on the filters, glass filters were weighed before and after preparation using an ultra-micro balance (MT5, Mettler-Toledo GmbH, Germany). The masses and stoichiometric amounts of KI and Na2S2O3 deposited on the filters were calculated and assessed using statistical tests. Additionally, the theoretical ozone removal potential of both scrubber types was compared.
2.4.2 Ozone breakthrough
Ozone was produced photo-chemically using a stable ozone generator (Analytik Jena GmbH & Co. KG, Germany) and continuously quantified with an APOA-370 air pollution monitor (HORIBA Europe GmbH, Germany). Relative humidity was monitored using a Testo 435-4 multifunction indoor air quality meter (Testo SE & Co. KG, Germany). To evaluate the breakthrough time of both scrubber materials under different conditions, 10 prepared glass filters and copper wool were placed in a 3D-printed filter holder (Fig. S1 in the Supplement), which was designed to meet the flow requirements (>600 mL min−1) of the ozone monitor and to ensure a homogeneous flow through all filters (80 mL min−1 each). Relative humidity and ozone concentration were adjusted by combining flows of dry, humid, and ozone-containing air using mass flow controllers. The filter holder was placed between the outlet of the combined airflow and the ozone analyzer. Breakthrough tests for both scrubber materials and copper wool at a constant input ozone concentration of 50±3 ppb and varying relative humidity values between 1 % and 90 % at 20–23 °C were performed over the course of 10 h (Table S1 in the Supplement).
2.4.3 Long-term stability test
In order to assess the long-term stability and ozone reduction capability of both scrubber materials during a field sampling campaign, 20 freshly prepared ozone filters of each scrubber material were placed horizontally in a 3D-printed filter holder plate. The plate was then positioned horizontally inside a sampler box and exposed to a continuous flow of ambient air across the filter's surface, simulating the exposure during a real sampling scenario. Ozone concentration, temperature, relative humidity, and barometric pressure were monitored during the exposure experiment (Table S2). After 5 and 10 d, respectively, 10 filters per scrubber material were transferred to the laboratory, and the breakthrough experiment according to Sect. 2.4.2 was performed with an input ozone concentration of 50 ppb and a relative humidity of 60 %. The removal efficiency of the exposed filters was compared to the freshly prepared ozone scrubbers.
2.5 Load-and-flush method
Samples were loaded onto the bio-monitoring sorbent tubes by injecting 20 µL of a standard solution containing α-pinene, limonene, linalool, and myrcene with concentrations of 1.13, 1.10, 0.99, and 1.00 ng µL−1, respectively, using a cooled injection system (CIS; Gerstel GmbH & Co. KG, Germany) with a start temperature of 120 °C and heating up to 200 °C. A continuous flow of 100 mL min−1 through the injector and sorbent tube was realized for 5 min using an air sampling pump (AMA Instruments GmbH, Germany). The resulting masses of the analytes per sorbent tube were 22.6 ng of α-pinene, 22.0 ng of limonene, 19.9 ng of linalool, and 20.0 ng of myrcene. Triplicates of loaded sorbent tubes were placed in the sample extraction setup (Fig. S2) and flushed for 4 h with a flow of 80 mL min−1 with air at different levels of ozone concentration and relative humidity (Table S3). Each sample was additionally loaded with 50 ng of bromobenzene-D5 as an internal standard. Two additional loaded sorbent tubes per combination of ozone concentration and relative humidity were placed in the sample introduction system without the ozone scrubber as reference samples.
2.6 Permeation method
An adapted sample introduction system (Mayer and Borsdorf, 2014) was used to generate air samples containing different quantities of analytes, levels of relative humidity, and ozone concentrations (Fig. S2). For each substance, a permeation vessel was prepared by adding about 500 µL of the substance into a GC vial and closing it with a polyethylene membrane. The permeation vessel containing linalool was additionally equipped with a perforated septum to lower the permeation rate. The four permeation vessels were placed pairwise in two glass tubes, which were continuously flowed through with nitrogen (5.0 grade) at a rate of 500 mL min−1. Defined partial flows from both glass tubes were introduced into a mixing chamber via needle valves and mass flow meters. An additional gas flow of nitrogen (5.0 grade) was introduced via a mass flow controller for further dilution of the gas stream. An aliquot of the combined and diluted gas stream was transferred to a second mixing chamber using a needle valve and a mass flow meter. In this second mixing chamber, the substance-containing gas stream was further diluted with humidified and ozone-containing air. Parts of the final combined airstream were continuously sucked through the sorbent tubes for 4 h with a constant flow of 80 mL min−1 using a sample extraction setup containing mass flow controllers and a vacuum pump. Temperature, relative humidity, and ozone concentrations of the final gas stream were measured before and after the enrichment experiments (Table S4). The masses of all reference substances enriched per sample were calculated based on their permeation rates, the dilution setup, and the total volume of enriched air, resulting in mean values (± SD) of 21.58 ± 1.93 ng of α-pinene, 27.43 ± 2.02 ng of limonene, 29.52 ± 8.74 ng of linalool, and 24.71 ± 1.97 ng of myrcene. Permeation rates were calculated based on the mass losses of each permeation vessel over a time of at least 24 h. All used mass flow controllers and mass flow meters were calibrated using a bubble flow meter before use.
2.7 Application of potassium iodide ozone scrubbers under real-world conditions
Two MTS-32 multi-tube sequential samplers (Markes International Ltd., UK) were operated in parallel at the SMEAR II site at the Hyytiälä Forestry Field Station (Finland) for a period of 2 consecutive days in September 2024. Forest air was enriched using bio-monitoring sorbent tubes (Markes International Ltd., UK) for 4 h per sample and a flow of 80 mL min−1 above canopy level at 35 m height. In order to evaluate the influence of the ozone scrubber, one sampler was operated with KI-loaded filters and one without ozone filters. To ensure comparability, both samplers were operated using the same parameters (sampling time of 240 min, flow rate of 80 mL min−1), and the flow through all tube positions was verified to be equal (±5 %) using a flow meter (7000 GC flowmeter, Ellutia Ltd., UK). Ozone concentration, temperature, relative humidity, and precipitation were monitored during the sampling period using the SMEAR II site's monitoring infrastructure and extracted from the SmartSMEAR database (Junninen et al., 2009).
2.8 TD–GC–MS analysis
A GC–MS system consisting of an 8890 gas chromatograph and a 5977C single-quadrupole mass spectrometer (Agilent Technologies, Inc., USA) was used for terpene analysis. A TD100-xr thermal desorption system (Markes International Ltd., UK) was used to introduce the samples into the instrument. The sample tube was dry-purged before desorption for 2 min with a flow of 20 mL min−1 to remove water. Tube desorption onto a focusing trap (general purpose, Markes International Ltd., UK) was performed at 300 °C for 5 min with a flow of 100 mL min−1. The focusing trap was purged for 1 min with a purge flow of 20 mL min−1 at 20 °C and finally desorbed at 300 °C for 5 min with a column flow of 0.75 mL min−1 and a split flow of 5 mL min−1. Chromatographic separation was done using a VF-5ms column (40 m ×0.15 mm ID, 0.15 µm, Agilent Technologies, Inc., USA) with hydrogen as a carrier gas, a column flow of 0.75 mL min−1, and the following temperature program: 35 °C for 6.5 min, 6 °C min−1 to 210 °C, 25 °C min−1 to 280 °C, 280 °C for 2 min. Mass spectrometric detection was done using an electron impact ionization (EI) source operated at 70 eV and 230 °C. The quadrupole was operated in scan mode for 35–500 at 150 °C. External calibration of the GC–MS system was done using a reference standard mixture. All analytes were calibrated in a range of 1.5–30 ng per sorbent tube. Calibration standards were prepared by injecting a maximum of 20 µL methanolic standard solutions on bio-monitoring sorbent tubes according to the procedure described in Sect. 2.5. The performance of the TD–GC–MS system was periodically checked using a weighed reference standard mixture.
2.9 Data analysis
GC–MS data were analyzed using MassHunter quantitative analysis (v. 12.0.893.1, Agilent Technologies, Inc., USA). Further data analysis, statistical tests, and data visualization were performed in RStudio (v. 2023.12.1+402, RStudio Team, 2020) using R (v. 4.2.2, R Core Team, 2022) with the packages ggplot2 (v 3.5.0, Wickham, 2016), ggpubr (v. 0.6.0, Kassambara, 2023), scales (v. 1.3.0, Wickham et al., 2023b), ggbreak (v. 0.1.2, Xu et al., 2021), tidyr (v. 1.3.1, Wickham et al., 2024), lme4 (v. 1.1-35.5, Bates et al., 2015), emmaeans (v. 1.10.5, Lenth, 2024) and dplyr (v. 1.1.4, Wickham et al., 2023a). The design of the 3D-printed components and ozone scrubbers, as well as the generation of rendered images, were conducted using the software Autodesk Inventor Professional 2004.3 (Autodesk Inc., USA).
3.1 Characterization of ozone filters
The mass of scrubber material deposited on the filters after drying was 7.57 ± 0.82 mg (0.046 ± 0.005 mmol) for KI and 9.54 ± 0.86 mg (0.038 ± 0.003 mmol) for Na2S2O3 ⋅ 5 H2O (Table S5). Independent two-sided t tests were performed to assess differences between both scrubber types, revealing statistically significant differences in the masses (p<0.001, n=20) and stoichiometric amounts (p<0.01, n=20) of the scrubber materials deposited on the glass filters. The mean mass of KI deposited on the glass filters was significantly lower than that of Na2S2O3, but the stoichiometric amount of KI was significantly higher due to its lower molar mass. These results indicate a higher theoretical ozone removal potential of the KI ozone scrubber, based on the reactions of both materials with ozone (Reaction R1 (Helmig, 1997) and Reaction R2 (Deal et al., 2024; Ernle et al., 2023)).
To assess the ozone removal potential, the breakthrough of ozone for both filter materials was monitored over the course of 10 h, which was chosen to reflect realistic sampling durations commonly used in field applications. This duration ensures that the performance of the filters is evaluated under conditions that closely resemble practical usage while also covering sufficient time to observe the increase in the downstream ozone concentration over time. An input ozone concentration of 50 ± 3 ppb was used, with different levels of relative humidity tested to assess its influence on the ozone removal efficiency (Fig. 2).
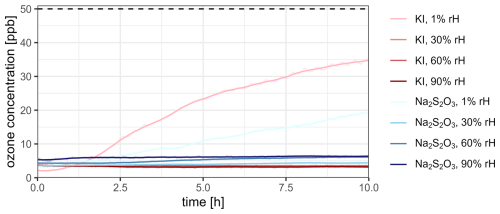
Figure 2Progression of the ozone concentration downstream of the ozone scrubbers at different relative humidity levels and an input ozone concentration of 50 ± 3 ppb (dashed horizontal line) and a flow rate of 80 mL min−1 per filter. Potassium iodide (KI, red graphs) and sodium thiosulfate (Na2S2O3, blue graphs) were used as scrubber materials.
At a very low relative humidity level, both scrubber materials showed an increase in ozone concentration downstream of the filters in comparison to higher relative humidity levels. This demonstrates that a very low relative humidity inhibits the reaction of both ozone scrubber materials with ozone, consecutively reducing the ozone removal potential. This diminished ozone removal potential can be explained by the role of water in both scrubber–ozone reactions. While water is a reactant in the reaction of ozone and KI (Reaction R1), the depletion of ozone with Na2S2O3 likely takes place in an aqueous medium (Deal et al., 2024). Therefore, the presence of water is necessary to facilitate both ozone depletion reactions. Relative humidity levels above 30 % result in downstream ozone concentrations of less than 7 ppb, which corresponds to removal efficiencies above 85 %. These results are consistent with a previous study, showing that humidity has an influence on the scrubber lifetime of Na2S2O3, with consistently higher ozone removal efficiencies at elevated humidity levels compared to dry air (Ernle et al., 2023). The highest ozone removal potential was observed for KI, with an average of approximately 93 % across all humidity levels. A linear model, formulated as “ozone concentration ∼ material * humidity * exposure time”, was applied to the dataset to investigate the influence of scrubber material, relative humidity, and time on the downstream ozone concentration. In order to ensure that the model reflected realistic environmental conditions, the lowest humidity level was omitted. The analysis demonstrated that both exposure time and humidity significantly affect the downstream ozone concentration, with differing impacts between the two scrubber materials. For Na2S2O3, a 1 % increase in relative humidity led to a rise in ozone concentration by 0.0355 ppb (p<0.0001). In contrast, this effect was significantly smaller for KI (−0.0398 ppb per 1 % increase, p<0.0001), resulting in an almost negligible net effect (−0.043 ppb per 1 % rH increase). This indicates that KI scrubbers are largely unaffected by changes in humidity, while Na2S2O3 scrubbers experience a notable decline in performance with increasing humidity. Similarly, exposure time increased ozone concentration for Na2S2O3 by 0.1467 ppb h−1 (p<0.0001), whereas the compared effect was significantly less pronounced for KI (−0.1525 ppb h−1, p<0.0001), resulting in a near-zero net effect of −0.0058 ppb h−1. This highlights the ability of KI scrubbers to effectively prevent ozone build-up over time. Second-order interactions between humidity and time, as well as the three-way interaction with scrubber material, were not statistically significant, indicating no substantial combined effects on the downstream ozone concentration. The model demonstrated an excellent fit, with a residual standard error of 0.1918 and an adjusted R2 value of 0.9685, explaining 96.85 % of the variance in ozone concentration. The high F statistic (5294; ) confirms the model's robustness.
Additionally, a long-term stability test was performed to evaluate the influence of exposure to ambient air on the scrubber's performance. Therefore, the filters were exposed to ambient air with varying ozone concentrations between 6 and 67 ppb and relative humidity levels ranging from 44 % to 84 % for 5 and 10 d, representing both typical environmental conditions and time frames of a sampling campaign (Table S2). After exposure, the breakthrough of ozone was monitored using the same approach as for the freshly prepared filters (Fig. 3).
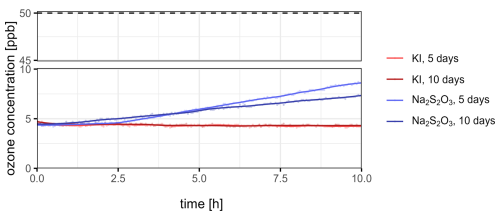
Figure 3Progression of the ozone concentration downstream of the ozone scrubbers at 60 % relative humidity and an input ozone concentration of 50±3 ppb (dashed horizontal line) and a flow rate of 80 mL min−1 per filter over the course of 10 h. Potassium iodide (KI, red graphs) and sodium thiosulfate (Na2S2O3, blue graphs) scrubbers were stored under environmental conditions for 5 and 10 d before the breakthrough tests.
The measured downstream ozone concentration after 5 and 10 d of exposure to ambient air remained at a consistently low level for the KI-loaded filters, demonstrating an ozone removal efficiency of over 90 % comparable to freshly prepared filters. In contrast, the downstream ozone concentration of the Na2S2O3-loaded filters showed increase over time after both 5 and 10 d of exposure, leading to a diminished ozone removal efficiency of about 84 %. However, for short sampling times less than 2 h, the ozone removal efficiency still exceeded 90 %. These results indicate that, in contrast to Na2S2O3, the KI-loaded ozone scrubbers' efficiency is not significantly affected by exposure to ambient air for up to 10 d. This is in accordance with a previous study, showing a higher ozone removal potential of KI compared to Na2S2O3 (Fick et al., 2001). However, both scrubber materials show sufficiently high ozone removal efficiencies, as shown in previous studies, especially for short sampling durations (Ernle et al., 2023; Hellén et al., 2024).
Additionally, the influence of the copper wool on the downstream ozone concentration was investigated at an input ozone concentration of 50 ppb across different humidity levels over the course of 4 h using cleaned glass filters without any scrubber material (Fig. 4).
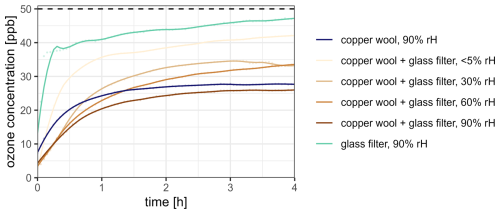
Figure 4Progression of the ozone concentration downstream of the copper wool and clean glass filters at different relative humidity levels and an input ozone concentration of 50 ± 3 ppb (dashed horizontal line) and a flow rate of 80 mL min−1 per filter over the course of 4 h, representing a typical sampling duration. Only copper wool (dark-blue graph) and only clean glass filters (light-blue line) were used as references.
Our results demonstrate that the used copper wool has an additional positive effect on the ozone removal potential of the scrubber. At a relative humidity level of 90 %, clean glass filters reduce the amount of ozone passing through by approximately 14 % over the course of 4 h. This reduction may be attributed to the adsorption and dissolution of ozone in water films forming within the porous structure of the glass filter (Willis and Wilson, 2022). Using only copper wool under the same conditions, the amount of ozone passing through is reduced by approximately 50 %, which indicates an ozone-depleting reaction at the surface of the copper wool. When combining copper wool and clean glass filters, the reduction in downstream ozone increases with rising relative humidity. At <5 % rH, ozone levels decrease by 28 %, while at 90 % rH, the reduction reaches 56 %. This suggests a humidity-dependent ozone removal mechanism, likely involving the formation of Cu2O by wet oxidation of Cu and further reaction between the Cu–Cu2O system and ozone (Reaction R3, Kim et al., 2023; Lin and Frankel, 2013; Ma et al., 2024).
This is supported by the optical changes observed after the breakthrough experiments at high relative humidity, where the copper surface lost its metallic luster and developed a faint, dull reddish-brown tint, indicating the formation of Cu2O. Furthermore, the 56 % reduction in downstream ozone observed with the combination of copper wool and a glass filter suggests a cumulative effect. The glass filter initially removes 14 % of the ozone, followed by an additional 50 % reduction by the copper wool, theoretically leading to a total removal of 57 % of the initial ozone passing through the system.
In summary, while both scrubber materials demonstrate efficacy, the KI-loaded ozone scrubbers demonstrated a slightly superior performance in ozone depletion compared to the Na2S2O3-loaded glass filters with consistent removal efficiencies >90 %, characterized by a lower initial downstream ozone concentration, a diminished increase in ozone concentration over time, a less pronounced influence of varying relative humidity levels during sampling, and a reduced propensity to be negatively affected by exposure to ambient air. These findings indicate that both ozone scrubber materials are generally capable of removing ozone from the sampled airstream across a range of relative humidity levels. Furthermore, the copper wool demonstrated an additional ozone-depleting effect, enhancing the overall ozone removal potential of the scrubber design. Therefore, the proposed scrubber design, where each sorbent tube is equipped with its own ozone filter, is especially suitable for long-term environmental monitoring using multi-tube samplers, since the exposure to ambient air of varying environmental conditions for a longer period of time does not diminish the ozone removal potential when KI is used. Consequently, there is no need for regular checks and replacement during a monitoring campaign, in contrast to other ozone filter designs (Hellén et al., 2024; Pollmann et al., 2005).
3.2 Influence of ozone scrubbers on terpene sampling under controlled laboratory conditions
To assess the influence of the ozone filter on the sampling of forest VOCs, α-pinene (bicyclic monoterpene), myrcene (acyclic monoterpene), limonene (monocyclic monoterpene), and linalool (acyclic monoterpene alcohol) were selected as reference compounds to cover a broad range of structural and chemical characteristics (Fig. S3). The influence of the filters on these compounds was evaluated using two different approaches: (a) a load-and-flush method, were the influence of humid ozone-containing air to already-adsorbed molecules is examined, and (b) a permeation method, which covers the gas-phase reaction of the analytes with ozone, the interaction of analytes with humid air and the scrubber material, and the possible decomposition of adsorbed substances by ozonation, representing a realistic sampling scenario. Experimental conditions comprised relative humidity levels of approximately 5 %, 30 %, 60 %, and 90 % combined with ozone concentrations of <6, 25, and 50 ppb for each scrubber material, yielding a total of 24 combinations for each approach. For each approach and each combination of ozone concentration, relative humidity level, and scrubber material, three filtered samples and one unfiltered sample were generated, resulting in a total of 192 samples. All experiments were conducted using “bio-monitoring” sorbent tubes, which contain a combination of Tenax TA and Carbograph 5TD as sorbent material. This combination offers the advantages of capturing a broader range of VOCs compared to individual sorbents. The polymer-based Tenax TA is particularly effective for medium- to high-molecular-weight VOCs, like mono- and sesquiterpenes, while the carbon-based sorbent Carbograph 5TD efficiently adsorbs very light and low-molecular-weight VOCs, like isoprene. This combination is therefore especially suitable for the monitoring of forest environments, where a broad range of volatile organic compounds is present (Borsdorf et al., 2023; Hellén et al., 2018; Jaakkola et al., 2024).
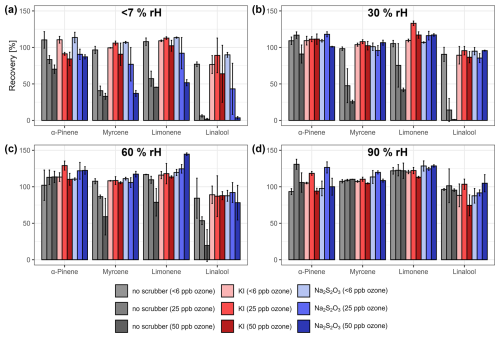
Figure 5Mean recovery rates (± standard deviation) of α-pinene, myrcene, limonene, and linalool for the load-and-flush approach at relative humidity levels of (a) (b) 30 %, (c) 60 %, and (d) 90 %. Gray bars represent unfiltered samples, red bars potassium iodide filters, and blue bars sodium thiosulfate filters.
3.2.1 Load-and-flush approach
Using the load-and-flush approach, the influence of the ozone scrubbers on the recovery rates of already-adsorbed analytes was compared across different ozone concentrations and relative humidity levels with and without the use of an ozone scrubber (Fig. 5).
Without an ozone filter and at low relative humidity values, the recovery of all substances decreased as ozone concentrations increased, following the order α-pinene < limonene < myrcene < linalool, with linalool showing the highest recovery loss. This reduction can be attributed to the reaction of adsorbed analytes with ozone passing the sorbent tube, resulting in analyte loss. The findings align closely with the predicted reactivity of the analytes with ozone based on their chemical structures and gas-phase reaction constants, which here serve as a reference for analyte decomposition on the sorbent material (Bernard et al., 2012; IUPAC, 2024, Fig. S3). The degree of decomposition of the substances is expected to increase with the number of double bonds, while terminal double bonds are less affected than internal double bonds, resulting in the order α-pinene < limonene < linalool < myrcene (Calogirou et al., 1996). While our results are in accordance with the expectations for α-pinene and limonene, linalool shows a slightly higher degree of decomposition compared to myrcene. This discrepancy may be attributed to the different nature of gas-phase reactions in comparison to the reaction of adsorbed substances. As humidity increases, the effect of increasing ozone concentrations is mitigated, indicating that a rise in humidity levels prevents ozone–analyte interactions, even in the absence of an ozone-depleting reagent. At 90 % rH, no reduction in recovery was observed with increasing ozone concentrations, indicating that the reaction of ozone with adsorbed analyte molecules is effectively inhibited. This demonstrates that the water content in the sampled airstream plays a positive role in preventing ozone–analyte interactions inside the sorbent tube. This can partly be attributed to the humidity-dependent ozone depletion by the copper wool but does not fully explain the high recovery rates at 90 % relative humidity.
When either of the scrubber materials was used, the recovery rates at the lowest humidity level also decreased with rising ozone concentrations, but to a generally lesser extent, which is in accordance with the observed reduced ozone removal efficiency of both scrubber materials under low relative humidity levels (Fig. 2). Comparing both scrubber materials, the loss of analytes was less pronounced for the KI scrubbers, exhibiting higher recovery rates for myrcene, limonene, and linalool, as well as comparable recovery rates for α-pinene. This is in contrast to the breakthrough experiments, which suggest a higher ozone removal potential of Na2S2O3 at low humidity. This may be explained by a lower relative humidity threshold for effectively filtering ozone of KI compared to Na2S2O3, as the breakthrough experiments were conducted at approximately 1 % rH, and the load-and-flush experiments were performed at around 5 % rH–6 % rH. When the relative humidity level exceeded 30 %, no decrease in the recovery rate of the four substances was observed with increasing ozone concentration. When aggregated across all tested relative humidity levels, no significant differences in recovery was observed between unfiltered samples at baseline ozone concentrations and filtered samples with either ozone scrubber across ozone concentrations up to 50 ppb (Wilcoxon rank sum test, p>0.01; Fig. S4a), indicating stable recovery values across a wide range of environmental conditions and therefore enhancing the measurement accuracy and fostering the inter-comparability of samples. However, their efficiency in preventing analyte loss is limited at very low relative humidity levels, consistent with the findings from the breakthrough experiments and a previous study conducted on Na2S2O3 (Ernle et al., 2023).
3.2.2 Permeation approach
Using the permeation method, the influence of the ozone scrubbers on the recovery rates was compared across different ozone concentrations and relative humidity levels both with and without the use of an ozone scrubber. This approach gives additional information about possible interactions between analytes in the gas phase and the scrubber material while passing the ozone filter at various relative humidity levels and simulates a real-world sampling scenario. Overall, the recovery rates of the investigated substances were below 100 %, except for α-pinene at <7 % rH and ozone concentrations <25 ppb (Fig. S5). This may be explained by systematic losses resulting from possible interactions between the analytes and the internal surface of the permeation device or the gas-phase interaction between analyte molecules and water or ozone before passing the ozone scrubber. Additionally, increasing humidity in the gas stream may saturate the sorbent material with water, blocking the binding sites and hindering adsorption of analytes, ultimately leading to an overall decreased recovery (Ho et al., 2017; Wilkinson et al., 2020). Furthermore, possible over- or underestimation of the calculated analyte concentrations in the airstream due to inaccuracies in the weighing method of the permeation vessels may lead to higher or lower recovery values. In order to mitigate these uncertain systematic errors, the recovery rates were normalized to the unfiltered samples at baseline ozone concentration for each humidity level (Fig. 6).
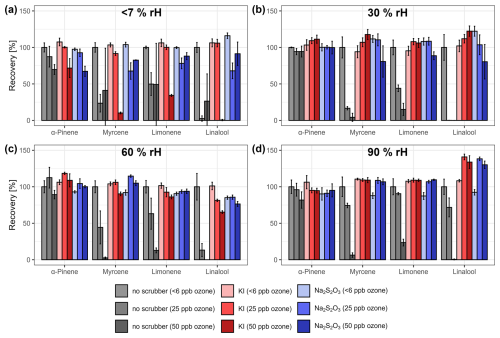
Figure 6Mean recovery rates (±standard deviation) of α-pinene, myrcene, limonene, and linalool for the permeation approach at relative humidity levels of (a) <7 %, (b) 30 %, (c) 60 %, and (d) 90 % normalized to the non-filtered baseline ozone sample for each humidity level. Gray bars represent unfiltered samples, red bars potassium iodide filters, and blue bars sodium thiosulfate filters.
When ozone is present in the sampled airstream, and no filter is used, the recovery rates of all substances, except for α-pinene at >30 % rH, exhibit a noticeable decreasing trend with increasing ozone concentrations aligning with the sequence observed in the load-and-flush approach (α-pinene > limonene > myrcene > linalool) and culminating in the complete loss of linalool at 50 ppb O3 across all humidity levels. With increasing relative humidity, the loss in recovery is mitigated, showing a similar trend compared to the load-and-flush approach, but to a generally lesser extent. This discrepancy may be explained by the additional gas-phase interactions between the analyte molecules and ozone, water vapor, and the internal surfaces of the permeation device or the glass filters.
At baseline ozone concentrations <6 ppb, no noticeable differences in recovery rates were observed between samples with and without the use of both scrubber materials across all humidity levels. These results indicate that there is no reaction between the analyte molecules and the ozone-depleting reagents, nor any obstruction of the sampled air's flow path caused by interaction between water vapor and the slightly hygroscopic scrubber materials at elevated relative humidity levels.
When ozone is introduced, and one of the scrubber materials was used, the recovery rates of all four substances were generally higher than or comparable to the unfiltered samples across all humidity levels. At an ozone concentration of 50 ppb and low relative humidity, the use of KI scrubbers led to lower recovery rates in comparison to Na2S2O3, which is the opposite of the load-and-flush approach, indicating the limited reliability and efficiency of both scrubber materials at low relative humidity levels <7 %. When aggregated across all humidity levels ≥ 30 %, no significant differences in recovery rates were observed between unfiltered samples at baseline ozone concentrations and filtered samples with either scrubber material and ozone concentrations up to 50 ppb (Wilcoxon rank sum test, p>0.01; Fig. S4b).
Our results clearly highlight the extent of the negative influence of ozone on the measurement accuracy, emphasizing the necessity of using a suitable ozone filter. Both KI and Na2S2O3 proved to be generally well-suited ozone scrubber materials for sampling BVOCs in forest air, as they do not exhibit any negative effect on the various terpenes and show no adverse impact on analyte retention across typical relative humidity levels present in forests. Furthermore, both scrubber materials ensure that the measurement results accurately reflect the actual terpene concentrations present during sampling, thereby enhancing the reliability of terpene monitoring in forest air under different and changing environmental conditions.
3.2.3 Comparison of both validation approaches
A combination of statistical methods was employed to assess the influence of ozone, relative humidity, and the ozone scrubbers on the recovery rates of α-pinene, myrcene, limonene, and linalool in both the permeation and the load-and-flush approaches. A linear model, defined as “recovery ∼ ozone * humidity * filter”, was used to determine the direction and magnitude of individual factors and interaction among these factors. Analysis of variance (ANOVA) was performed to partition the total variance and assess the statistical significance of the main factors and interactions. Additionally, Tukey's honest significant difference test (TukeyHSD) was applied to compare the effects of different scrubber materials (Table 1). Effect sizes were compared across both methods using the partial eta-squared (Fig. S6). This integrated statistical approach provides a comprehensive and robust evaluation of the factors affecting the recovery rates across both methods and serves as the foundation for comparing the two approaches in terms of their suitability for validating ozone scrubbers and assessing their impact on analyte recoveries.
Table 1Results of the statistical analysis evaluating the effects of ozone (O3), relative humidity (rH), ozone scrubbers, and their interactions on the recovery rates of α-pinene, myrcene, limonene, and linalool in both the permeation and load-and-flush approaches. The upper section presents the outcomes of the linear model (recovery ∼ ozone * humidity * filter) and ANOVA, showing the directions of effects (increase ↑, decrease ↓, mixed effect ↕, and near-zero effect –) and their significance (, , , p< 0.1, and n.s. = not significant). The lower section summarizes the results of Tukey's honest significance difference test (TukeyHSD), comparing different scrubber materials.
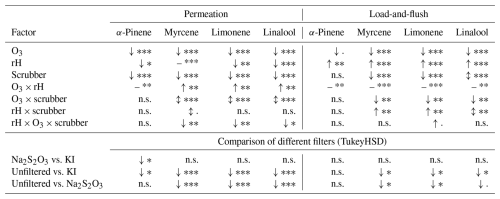
In both approaches, an increase in ozone concentration results in a highly significant decrease in the recovery of all analytes, except for α-pinene in the load-and-flush approach, where the effect is only marginally significant. This highlights the degradation of analytes due to ozonation, both during sampling and after adsorption on the sorbent material.
In contrast, increasing relative humidity leads to contrasting effects in the two methods. In the permeation approach, higher relative humidity leads to a significant decrease in recovery for all substances except myrcene, which remains largely unaffected. This effect can be attributed to the condensation of water vapor within the sorbent tube, forming a water layer on the sorbent material and consecutively inhibiting the access of analytes to the binding sites (Ho et al., 2017; Wilkinson et al., 2020). Conversely, in the load-and-flush approach, the recovery rates for all substances increase with rising humidity. This effect may also be explained by the formation of a water layer that inhibits the interaction of gas-phase ozone to the already-adsorbed analyte molecules, thereby mitigating ozone-induced degradation. Additionally, the effect size of relative humidity and interaction terms including humidity is considerably larger in the load-and-flush approach compared to the permeation method, indicating a stronger influence of humidity-related processes in this setup.
In the permeation method, the filters exhibit stronger and more statistically significant effects on the analyte recovery compared to the load-and-flush approach. This, along with the different effects of relative humidity on analyte recovery, suggests that the permeation method is better suited to evaluate the efficiency and suitability of the ozone scrubbers, since this method more closely reflects a real-world sampling scenario.
3.3 Influence of potassium iodide ozone scrubbers on terpene sampling under real-world conditions
Forest air was monitored for terpenes over the course of 48 h under changing environmental conditions. The temperature ranged from 12.9 to 20.4 °C, with relative humidity levels between 47 % and 100 % and ozone concentrations from 21 to 51 ppb. To evaluate the influence of the KI ozone scrubber, the measured terpene concentrations with and without the use of a scrubber were compared (Fig. 7a–c). Additionally, the peak areas of known Tenax degradation products for both scenarios were evaluated (Fig. 7d–f, Klenø et al., 2002).
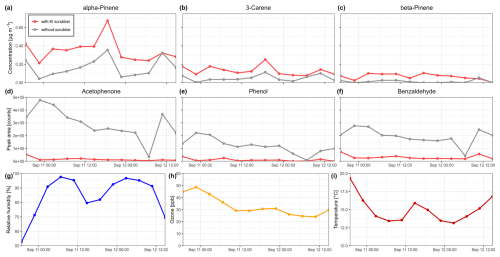
Figure 7Temporal variations in monoterpene concentrations (a–c), peak areas of Tenax degradation products (d–f), and environmental parameters (g–i) across the sampling period, using a potassium iodide ozone scrubber (red) and without the scrubber (gray). The results demonstrate that the ozone scrubber enhances the measured concentrations of forest monoterpenes and reduces the formation of degradation products of the sorbent material, indicating effective prevention of reactions of adsorbed monoterpenes and sorbent material with ozone.
The measured concentrations of monoterpenes (α-pinene, 3-carene, β-pinene) showed significant differences between filtered and unfiltered samples, with higher concentrations in filtered samples (Wilcoxon rank sum test, p<0.01). This demonstrates the positive effect of the used ozone scrubber on the measurement accuracy by preventing analyte loss due to interaction with ozone inside the sorbent tube, consistent with the findings from the laboratory experiments. No significant correlations between terpene concentrations and environmental parameters (humidity, temperature, ozone, precipitation, photosynthetically active radiation) were found. However, a spike in terpene concentrations can be sensed after temperature increased, and relative humidity levels decreased. 3-carene and α-pinene showed a significant positive correlation (ρ=0.91, p<0.05), indicating a similar emission pattern and dependency on environmental factors, as shown in a previous study (Borsdorf et al., 2023).
In contrast to the monoterpenes, the detected amounts of the Tenax degradation products acetophenone, phenol, and benzaldehyde were significantly higher in the unfiltered samples (Wilcoxon rank sum test, p<0.01), indicating the effective removal of ozone from forest air under varying conditions and preventing ozone–sorbent interactions. For the unfiltered samples, a Spearman correlation analysis revealed significant positive correlations of the degradation products with ozone, with correlation coefficients of 0.73 for acetophenone, 0.86 for phenol, and 0.60 for benzaldehyde (p<0.05), demonstrating the negative influence of ozone on the sorbent material.
These results demonstrate that the KI ozone scrubbers effectively enhance the quantification of terpenes in forest air under varying real environmental conditions while safeguarding the sorbent material from degradation. Thus, the results of the laboratory experiments could be confirmed under real-world conditions.
In conclusion, both KI and Na2S2O3 showed sufficient ozone removal performance across environmentally relevant levels of relative humidity and ozone concentration. A direct comparison between both scrubber materials showed that KI has a slightly better performance and is less affected by increasing relative humidity levels compared to Na2S2O3. Additionally, KI shows enhanced performance after exposure to ambient air for up to 10 d, with ozone removal efficiencies >90 %, which proves its long-term stability and demonstrates that regular inspections and replacement during longer monitoring campaigns is not necessary. No negative effect of either scrubber material on four structurally different monoterpenes (α-pinene, myrcene, limonene, linalool) was observed across different relative humidity levels and ozone concentrations using a load-and-flush and a permeation approach, demonstrating the suitability for monitoring of terpenes in forest air. At ozone concentrations of 25 and 50 ppb, the use of both ozone scrubber types resulted in recovery rates comparable to measurements without a filter at baseline ozone concentrations and the same relative humidity level. Therefore, the use of the novel ozone filter increases measurement accuracy and improves the comparability of measurements under different environmental conditions. In a field test scenario, the KI-loaded scrubbers were demonstrated to enhance the detection of forest monoterpenes under environmental conditions while safeguarding the sorbent material. This results in more accurate measurements and an increased longevity of the sorbent due to the prevention of reactions of ozone with adsorbed analytes and the sorbent material. These findings, in conjunction with the compatibility of the ozone scrubber design with multi-tube samplers and the rapid and straightforward replacement combined with the reusability of the glass filters, render this filter design a very well-suited choice for the environmental monitoring of VOCs without safety concerns and the need for additional devices and power supply. Additional optimization of the filter design may be possible by introducing a drying agent between the ozone filter and the sorbent bed to also mitigate the negative effect of high relative humidity levels on the sorption efficiency (Maceira et al., 2017). Furthermore, the different effects of relative humidity in both the load-and-flush and the permeation approach demonstrate that the comparatively simple load-and-flush approach is not sufficient to assess the suitability of ozone filters for use in environmental monitoring.
Data are available upon request by contacting the corresponding author (robby.rynek@ufz.de).
The supplement related to this article is available online at https://doi.org/10.5194/amt-18-4103-2025-supplement.
RR: conceptualization, methodology, formal analysis, investigation, visualization, writing (original draft). TM: conceptualization, methodology, resources, visualization, writing (review and editing). HB: conceptualization, methodology, resources, funding acquisition, writing (review and editing).
The contact author has declared that none of the authors has any competing interests.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
The authors would like to thank Anne Kretzschmar (UFZ) for her assistance in conducting the laboratory experiments and the preparation of field experiments as well as the team of the UFZ workshop for building the ozone scrubber cases. Furthermore, we thank the personnel of the Hyytiälä Forestry Field Station, especially Lauri Ahonen and Ilona Ylivinkka, for their assistance during the field experiment and their effort in the maintenance of the instrumentation. Additionally, we acknowledge Petri Keronen and Pasi Kolari (both University of Helsinki) for the provision of environmental data.
This research has been supported by the German Federal Ministry of Food and Agriculture (BMEL) and the German Federal Ministry of the Environment, Nature Conservation, Nuclear Safety and Consumer Protection (BMUV) within the framework of the Forest Climate Fund (grant number 2220WK15B4). Additionally, this work has received financial support by the H2020 project eLTER PLUS, GA 871128, and has received support from the University of Helsinki at the Hyytiälä SMEAR II LTER site (https://deims.org/663dac80-211d-4c19-a356-04ee0da0f0eb, last access: 20 March 2025).
The article processing charges for this open-access publication were covered by the Helmholtz Centre for Environmental Research – UFZ.
This paper was edited by Yoshiteru Iinuma and reviewed by Junfeng Liu and two anonymous referees.
Bates, D., Mächler, M., Bolker, B., and Walker, S.: Fitting Linear Mixed-Effects Models Using lme4, J. Stat. Softw., 67, 1–48, https://doi.org/10.18637/jss.v067.i01, 2015.
Bernard, F., Daële, V., Mellouki, A., and Sidebottom, H.: Studies of the gas phase reactions of linalool, 6-methyl-5-hepten-2-ol and 3-methyl-1-penten-3-ol with O 3 and OH radicals, J. Phys. Chem. A, 116, 6113–6126, https://doi.org/10.1021/jp211355d, 2012.
Borsdorf, H., Bentele, M., Müller, M., Rebmann, C., and Mayer, T.: Comparison of Seasonal and Diurnal Concentration Profiles of BVOCs in Coniferous and Deciduous Forests, Atmosphere, 14, 1347, https://doi.org/10.3390/atmos14091347, 2023.
Calogirou, A., Larsen, B. R., Brussol, C., Duane, M., and Kotzias, D.: Decomposition of Terpenes by Ozone during Sampling on Tenax, Anal. Chem, 68, 1499–1506, https://doi.org/10.1021/ac950803i, 1996.
Deal, A. M., Prophet, A. M., Bernal, F., Saykally, R. J., and Wilson, K. R.: A Detailed Reaction Mechanism for Thiosulfate Oxidation by Ozone in Aqueous Environments, Environ. Sci. Technol., 58, 18959–18968, https://doi.org/10.1021/acs.est.4c06188, 2024.
Ernle, L., Ringsdorf, M. A., and Williams, J.: Influence of ozone and humidity on PTR-MS and GC-MS VOC measurements with and without a Na2S2O3 ozone scrubber, Atmos. Meas. Tech., 16, 1179–1194, https://doi.org/10.5194/amt-16-1179-2023, 2023.
Fick, J., Pommer, L., Andersson, B., and Nilsson, C.: Ozone removal in the sampling of parts per billion levels of terpenoid compounds: An evaluation of different scrubber materials, Environ. Sci. Technol., 35, 1458–1462, https://doi.org/10.1021/es0001456, 2001.
Guenther, A., Hewitt, C. N., Erickson, D., Fall, R., Geron, C., Graedel, T., Harley, P., Klinger, L., Lerdau, M., Mckay, W. A., Pierce, T., Scholes, B., Steinbrecher, R., Tallamraju, R., Taylor, J., and Zimmerman, P.: A global model of natural volatile organic compound emissions, J. Geophys. Res.-Atmos., 100, 8873–8892, https://doi.org/10.1029/94JD02950, 1995.
Guenther, A. B., Jiang, X., Heald, C. L., Sakulyanontvittaya, T., Duhl, T., Emmons, L. K., and Wang, X.: The Model of Emissions of Gases and Aerosols from Nature version 2.1 (MEGAN2.1): an extended and updated framework for modeling biogenic emissions, Geosci. Model Dev., 5, 1471–-1492, https://doi.org/10.5194/gmd-5-1471-2012, 2012.
Hallquist, M., Wenger, J. C., Baltensperger, U., Rudich, Y., Simpson, D., Claeys, M., Dommen, J., Donahue, N. M., George, C., Goldstein, A. H., Hamilton, J. F., Herrmann, H., Hoffmann, T., Iinuma, Y., Jang, M., Jenkin, M. E., Jimenez, J. L., Kiendler-Scharr, A., Maenhaut, W., McFiggans, G., Mentel, Th. F., Monod, A., Prévôt, A. S. H., Seinfeld, J. H., Surratt, J. D., Szmigielski, R., and Wildt, J.: The formation, properties and impact of secondary organic aerosol: current and emerging issues, Atmos. Chem. Phys., 9, 5155–5236, https://doi.org/10.5194/acp-9-5155-2009, 2009.
Hellén, H., Kuronen, P., and Hakola, H.: Heated stainless steel tube for ozone removal in the ambient air measurements of mono- and sesquiterpenes, Atmos. Environ., 57, 35–40, https://doi.org/10.1016/j.atmosenv.2012.04.019, 2012.
Hellén, H., Praplan, A. P., Tykkä, T., Ylivinkka, I., Vakkari, V., Bäck, J., Petäjä, T., Kulmala, M., and Hakola, H.: Long-term measurements of volatile organic compounds highlight the importance of sesquiterpenes for the atmospheric chemistry of a boreal forest, Atmos. Chem. Phys., 18, 13839–13863, https://doi.org/10.5194/acp-18-13839-2018, 2018.
Hellén, H., Tykkä, T., Schallhart, S., Stratigou, E., Salameh, T., and Iturrate-Garcia, M.: Measurements of atmospheric C10–C15 biogenic volatile organic compounds (BVOCs) with sorbent tubes, Atmos. Meas. Tech., 17, 315–333, https://doi.org/10.5194/amt-17-315-2024, 2024.
Helmig, D.: Ozone removal techniques in the sampling of atmospheric volatile organic trace gases, Atmos. Environ., 31, 3635–3651, https://doi.org/10.1016/S1352-2310(97)00144-1, 1997.
Helmig, D., Revermann, T., Pollmann, J., Kaltschmidt, O., Hernández, A. J., Bocquet, F., and David, D.: Calibration system and analytical considerations for quantitative sesquiterpene measurements in air, J. Chromatogr. A, 1002, 193–211, https://doi.org/10.1016/S0021-9673(03)00619-8, 2003.
Ho, S. S. H., Chow, J. C., Watson, J. G., Wang, L., Qu, L., Dai, W., Huang, Y., and Cao, J.: Influences of relative humidities and temperatures on the collection of C2-C5 aliphatic hydrocarbons with multi-bed (Tenax TA, Carbograph 1TD, Carboxen 1003) sorbent tube method, Atmos. Environ., 151, 45–51, https://doi.org/10.1016/j.atmosenv.2016.12.007, 2017.
Hwan Lee, J., Batterman, S. A., Jia, C., and Chernyak, S.: Ozone artifacts and carbonyl measurements using tenax GR, tenax TA, carbopack B, and carbopack X adsorbents, J. Air Waste Manage., 56, 1503–1517, https://doi.org/10.1080/10473289.2006.10464560, 2006.
IUPAC: Task Group on Atmospheric Chemical Kinetic Data Evaluation, https://iupac.aeris-data.fr/en/home-english/, last access: 19 August 2024.
Jaakkola, E., Hellén, H., Olin, S., Pleijel, H., Tykkä, T., and Holst, T.: Ozone stress response of leaf BVOC emission and photosynthesis in mountain birch (Betula pubescens spp. czerepanovii) depends on leaf age, Plant-Environment Interactions, 5, e10134, https://doi.org/10.1002/pei3.10134, 2024.
Junninen, H., Lauri, A., Keronen, P., Aalto, P., Hiltunen, V., Hari, P., and Kulmala, M.: Smart-SMEAR: on-line data exploration and visualization tool for SMEAR stations, Boreal Env. Res., 14, 447–457, 2009.
Kassambara, A.: ggpubr: “ggplot2” Based Publication Ready Plots [code], https://rpkgs.datanovia.com/ggpubr/ (last access: 28 May 2024), 2023.
Kim, Y. J., Kim, D., Kim, Y., Jeong, Y., Jeong, B., and Park, J. Y.: Effect of Water Vapor on Oxidation Processes of the Cu(111) Surface and Sublayer, Int. J. Mol. Sci., 24, 810, https://doi.org/10.3390/ijms24010810, 2023.
Klenø, J. G., Wolkoff, P., Clausen, P. A., Wilkins, C. K., and Pedersen, T.: Degradation of the adsorbent tenax TA by nitrogen oxides, ozone, hydrogen peroxide, OH radical, and limonene oxidation products, Environ. Sci. Technol., 36, 4121–4126, https://doi.org/10.1021/es025680f, 2002.
Laothawornkitkul, J., Taylor, J. E., Paul, N. D., and Hewitt, C. N.: Biogenic volatile organic compounds in the Earth system, New Phytol., 183, 27–51, https://doi.org/10.1111/j.1469-8137.2009.02859.x, 2009.
Lenth, R. V: emmeans: Estimated Marginal Means, aka Least-Squares Means, CRAN [code], https://CRAN.R-project.org/package=emmeans, last access: 30 October 2024.
Lin, H. and Frankel, G. S.: Atmospheric corrosion of Cu by UV, ozone and NaCl, Corros. Eng. Sci. Techn., 48, 461–468, https://doi.org/10.1179/1743278213Y.0000000104, 2013.
Maceira, A., Vallecillos, L., Borrull, F., and Marcé, R. M.: New approach to resolve the humidity problem in VOC determination in outdoor air samples using solid adsorbent tubes followed by TD-GC–MS, Sci. Total Environ., 599–600, 1718–1727, https://doi.org/10.1016/j.scitotenv.2017.05.141, 2017.
Ma, G., Guan, J., Zhu, Q., Jiang, Y., Han, N., and Chen, Y.: A Review of Ozone Decomposition by a Copper-Based Catalyst, Catalysts, 14, 264, https://doi.org/10.3390/catal14040264, 2024.
Mayer, T. and Borsdorf, H.: Accuracy of Ion Mobility Measurements Dependent on the Influence of Humidity, Anal. Chem., 86, 5069–5076, https://doi.org/10.1021/ac5007393, 2014.
Mermet, K., Sauvage, S., Dusanter, S., Salameh, T., Léonardis, T., Flaud, P.-M., Perraudin, É., Villenave, É., and Locoge, N.: Optimization of a gas chromatographic unit for measuring biogenic volatile organic compounds in ambient air, Atmos. Meas. Tech., 12, 6153–6171, https://doi.org/10.5194/amt-12-6153-2019, 2019.
Pollmann, J., Ortega, J., and Helmig, D.: Analysis of atmospheric sesquiterpenes: Sampling losses and mitigation of ozone interferences, Environ. Sci. Technol., 39, 9620–9629, https://doi.org/10.1021/es050440w, 2005.
R Core Team: R: A Language and Environment for Statistical Computing [software], https://www.R-project.org/, last access: 23 May 2024.
RStudio Team: RStudio: Integrated Development Environment for R [software], http://www.rstudio.com/, last access: 31 Janury 2024.
Sindelarova, K., Granier, C., Bouarar, I., Guenther, A., Tilmes, S., Stavrakou, T., Müller, J.-F., Kuhn, U., Stefani, P., and Knorr, W.: Global data set of biogenic VOC emissions calculated by the MEGAN model over the last 30 years, Atmos. Chem. Phys., 14, 9317–9341, https://doi.org/10.5194/acp-14-9317-2014, 2014.
Wickham, H.: ggplot2: Elegant Graphics for Data Analysis, Springer-Verlag New York, ISBN 978-3-319-24277-4, https://ggplot2.tidyverse.org (last access: 24 February 2024), 2016.
Wickham, H., François, R., Henry, L., Müller, K., and Vaughan, D.: dplyr: A Grammar of Data Manipulation, https://CRAN.R-project.org/package=dplyr (last access: 28 May 2024), 2023a.
Wickham, H., Pedersen, T. L., and Seidel, D.: scales: Scale Functions for Visualization, https://CRAN.R-project.org/package=scales (last access: 28 May 2024), 2023b.
Wickham, H., Vaughan, D., and Girlich, M.: tidyr: Tidy Messy Data, https://CRAN.R-project.org/package=tidyr, (last access: 28 May 2024), 2024.
Wilkinson, M., White, I. R., Goodacre, R., Nijsen, T., and Fowler, S. J.: Effects of high relative humidity and dry purging on VOCs obtained during breath sampling on common sorbent tubes, J. Breath Res., 14, 046006, https://doi.org/10.1088/1752-7163/ab7e17, 2020.
Willis, M. D. and Wilson, K. R.: Coupled Interfacial and Bulk Kinetics Govern the Timescales of Multiphase Ozonolysis Reactions, J. Phys. Chem. A, 126, 4991–5010, https://doi.org/10.1021/acs.jpca.2c03059, 2022.
Xu, S., Chen, M., Feng, T., Zhan, L., Zhou, L., and Yu, G.: Use ggbreak to Effectively Utilize Plotting Space to Deal With Large Datasets and Outliers, Front. Genet, 12, https://doi.org/10.3389/fgene.2021.774846, 2021.




