the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Correcting for water vapor diffusion in air bag samples for isotope composition analysis: case study with drone-collected samples
Di Wang
Camille Risi
Lide Tian
Di Yang
Gabriel J. Bowen
Siteng Fan
Hongxi Pang
Laurent Z. X. Li
Mass spectrometry and laser spectroscopy have been widely employed for precise water vapor isotope measurements. Nevertheless, these techniques are limited by logistical challenges in fieldwork, consequently constraining the temporal and spatial resolution of measurements. Specifically, water vapor isotope measurements are primarily limited to near-surface levels, while measurements in the free troposphere are notably scarce. Portable sampling devices, such as air bags and glass bottles, have therefore become necessary alternatives for collecting, storing, and transporting gaseous samples in diverse environments prior to analysis with less portable instruments. In drone-based high-altitude vapor sampling, air bags are preferred for their lighter weight and greater flexibility compared to glass bottles. Nevertheless, they present specific challenges, such as potential sample contamination and isotopic fractionation during storage, primarily due to the inherent permeability of air bags. Here, we developed a theoretical model for water vapor diffusion through the sampling bag surface, with parameters calibrated through laboratory experiments. This model enables the reconstruction of the initial isotopic composition of sampled vapor based on measurements obtained within the bag and from the surrounding environment. This diffusion model underwent rigorous validation through experiments conducted under varying humidity and isotopic composition differences between the inside and outside of the air bag, confirming its reliability. We applied this correction method to air samples collected at various pressures up to the upper troposphere using an air bag-mounted drone that we developed, thereby estimating the initial isotopic composition and uncertainty based on our observations. Our correction method enhances the reliability and applicability of water vapor isotope observations conducted using drones equipped with air bags, and provides a detailed assessment of all potential sources of error and quantifies the uncertainty range of the observations. This approach leverages the strengths of drone-based air bag sampling while mitigating its limitations, thus facilitating the convenient collection of isotopic data throughout the troposphere.
- Article
(4531 KB) - Full-text XML
- BibTeX
- EndNote
Water vapor isotopes provide unique insights into the transport, mixing, and phase changes of water in the environment, which are crucial for improving understanding of the climate system, hydrological cycle, atmospheric dynamics, and paleoclimate proxies. Water isotopes have also been applied in climate modeling, weather prediction, and water resource management (Bowen et al., 2019; Gat, 1996; Galewsky et al., 2016; West et al., 2009).
Water isotope analysis has traditionally relied on mass spectrometry, which, while accurate (Ghosh and Brand, 2003; Muccio and Jackson, 2009), demands labor-intensive preparation and lacks portability (West et al., 2010). Methods like cryogenic trapping effectively collect water vapor (Michener and Lajtha, 2008; Yu et al., 2015; Grootes and Stuiver, 1997; Steen-Larsen et al., 2011), but they require long sampling periods, limiting observation scope and timing. Over the past three decades, laser spectroscopy methods such as Cavity Ring-Down Spectroscopy (CRDS) (Hodges and Lisak, 2006) and Off-Axis Integrated Cavity Output Spectroscopy (OA-ICOS) (Johnson et al., 2011) have emerged, delivering a best-in-class combination of speed, high precision, and continuous measurements even in challenging environments such as high altitudes or arid regions with low water vapor content. Advances in these instruments have significantly expanded the field of water isotope research. However, their heavy instrumentation, substantial power requirements, and limited mobility restrict their usability in certain situations, particularly for aerial water vapor isotope measurements, which require lightweight and flexible sampling approaches. As a result, the collection and storage of physical samples are still necessary, increasing the demand for more convenient and efficient sample acquisition methods. Air bags and glass bottles have been practical solutions for collecting, storing, and transporting gaseous samples from various settings (Rozmiarek et al., 2021).
Given that air bags can reduce the weight of sampling equipment and increase sampling flexibility, there is considerable interest in using them for vapor sample collection. This is particularly advantageous for small equipment like drones, where minimizing payload weight is essential for sampling at high altitudes or over long distances. This selection also makes it easier to transport samples and reduces the risk of breakage. However, concerns have arisen regarding the suitability of various sampling materials for storing these samples, primarily due to potential water diffusion through container walls. Diffusion issues are commonly observed in sampling bags during water vapor isotope analysis and have persisted as a longstanding challenge in the field (Herbstritt et al., 2023; Havranek et al., 2020; Magh et al., 2022; Gralher et al., 2021). In previous studies, this issue is particularly concerning in the Direct Vapor Equilibrium Laser Spectroscopy (DVE-LS) method, which has been widely used to rapidly collect and measure water isotopes in evaporation-prone soil, rock, or plant samples. The DVE-LS method simplifies preparation and increases sample throughput by directly analyzing the vapor phase, thus eliminating the need for extensive physical extractions (Gralher et al., 2021; Wassenaar et al., 2008; Hendry et al., 2015; Millar et al., 2018; Sprenger et al., 2015). However, water vapor molecules may exchange between the air inside the bag and the external ambient air during sample storage. Water vapor molecules typically diffuse from areas of high humidity to drier areas. In this process, heavier isotopologues (e.g., HO and HDO) move more slowly than lighter isotopologues (e.g., HO) due to their greater mass, resulting in preferential diffusion of lighter isotopes. This differential diffusion, can alter the original isotopic composition of the collected air samples. Moreover, differential diffusion can also occur due to gradients in isotopic composition.
To mitigate these issues, specific diffusion-tight materials are highly recommended for water vapor isotope measurements, although adsorption effects may still occur with such materials (Herbstritt et al., 2023). Further research and development are still necessary. This may involve exploring alternative materials for more impermeable sampling bags, improving sealing methods to better isolate sampled air, and developing sampling techniques less susceptible to diffusion. Resolving these issues is essential for ensuring the reliability of water vapor isotope measurements using air bags and for accurately understanding atmospheric and hydrological processes.
In light of the ongoing development and further refinement of these techniques and the associated cost constraints, we developed a diffusion model with parameters calibrated through laboratory experiments. This model is capable of assessing the permeability of the air bags and correcting the obtained isotope measurements to the initial pre-diffusion values based on the humidity, isotope values inside and outside the bag, and the sample storage time. This diffusion model was validated through experiments under varying humidity and isotopic composition differences between the inside and outside of the air bag, confirming its reliability. Furthermore, we also applied this diffusion method to air samples collected at different altitudes using a drone-based atmospheric vapor sampling device we developed, to estimate the initial isotope composition and uncertainty. The primary objective of this drone-based field campaign is to obtain atmospheric water vapor isotope data along vertical profiles in the troposphere, providing higher temporal and spatial resolution than satellite observations. The corrected near-surface drone-based measurements using our diffusion model show improved consistency with direct, in-situ surface-level measurements using the Picarro analyzer. Our model, validated by laboratory experiments under a wide range of environmental conditions, was used to assess all potential error sources and to quantify a conservative, comprehensive uncertainty range for the corrected vertical profiles. Although we minimized certain diffusion-related biases, such as pressure in different altitudes and long storage times, temperature and the pressure difference between the inside and outside of the bags on bag permeability were only addressed indirectly via conservative uncertainty estimates. This treatment is justified under our experimental conditions, which are characterized by the short residence time at high altitudes, the reduced diffusivity at low temperatures, and the flexibility of the bags maintaining equal internal and external pressure. Future applications should consider the specific experimental conditions, and future developments may extend the model to explicitly incorporate the dependence of bag permeability on partial pressure and temperature, thereby improving its applicability under a wider range of atmospheric conditions.
2.1 Diffusion model description
Storing vapor samples in air bags prior to isotope measurement may alter the isotopic composition of the water vapor. The main reason is the diffusion of water molecules between the interior and exterior of the air bags, primarily due to the permeability of the bag materials. We present a mathematical model for the diffusion and fractionation of isotopes across the surface of the sampling bag. In this model, we assume the ambient vapor flux entering the air bag changes the internal humidity and vapor isotopic composition, influenced by the different humidity and water isotopic composition inside and outside the bag (Fig. 1).
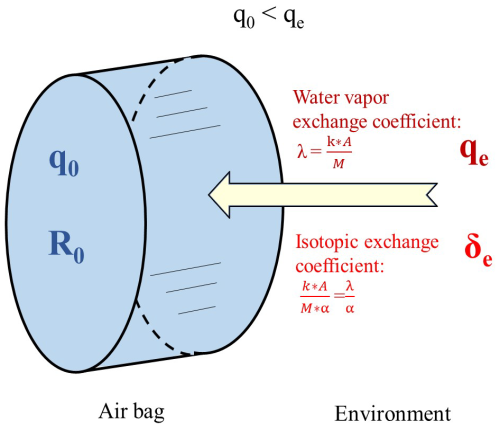
Figure 1Schematic illustrating the diffusion model. q0 represents the initial humidity in the air bag at t = 0, qe denotes the environmental humidity, R0 indicates the initial isotopic ratio in the air bag at t = 0, Re represents the isotopic ratio in the environment, k is water vapor conductance, A denotes the exchange area (surface area of the bag), and M is the mass of air within the bag, and α denotes the isotopic fractionation coefficient.
The flux of water into the bag, F (in kg m−2 s−1), is expressed as:
where q(t) represents the variation of humidity inside the air bag over time (in kg kg−1), qe denotes the environmental humidity (in kg kg−1), k is water vapor conductance. This first-order formulation is derived from Fick's diffusion law (Fick, 1855).
Similarly, the flux of isotopologue, Fi, either HO or HDO, moving into the bag can be described as:
In this equation, ki represents the conductance specific to each isotopologue (in kg m−2 s−1), Re denotes the isotopic ratio in the environment, and R(t) is the variation of isotopic ratio within the air bag with time. Notably, the fractionation coefficient here can be denoted as:
Since k represents the total conductance dominated by the lighter molecules (HO), and ki is defined as the conductance of the heavier isotopologues (either HO or HDO), Eq. (3) is therefore consistent with the conventional definition of fractionation factors ().
The validity of Eqs. (1) and (2) relies on the assumptions that internal and external pressures remain equal to atmospheric pressure, ensuring no pressure gradient across the bag membrane, that the internal vapor is well-mixed, and that the exchange rate follows a first-order process. Additionally, if the temperature remains constant, k and ki are assumed to be constant.
Assuming that diffusion through the bag is the primary transport mechanism, and neglecting adsorption, the temporal change in humidity can be modeled by the following differential equation:
where A represents the exchange area (surface area of the air bag), and M is the air mass inside the bag.
Assuming that M is constant, which is reasonable given that the total mass variation due to water vapor flux is at most 1 %, this equation simplifies to:
Here, we define the water vapor exchange coefficient as follows, it describes the rate at which water vapor exchanges across the bag membrane:
Similarly, the temporal change in isotopic ratio can be modeled by the following differential equation:
This equation can be simplified as:
If qe is stable (the environment is treated as an infinite reservoir), and that k and A are constant, the differential equation for humidity (Eq.`5) can be analytically solved:
where q0 is the initial humidity at t = 0. This equation can also be expressed in terms of natural logarithms as:
Consequently, the slope of ln (qe−q(t)) against time is λ.
For the isotopic ratio, the analytical solution is only feasible when the initial humidity equals the environmental humidity (q0=qe):
Assuming that Re, qe, k, α and A are constant.
Thus,
where R0 denotes the initial isotopic ratio at t = 0. Again, taking the natural logarithm, we obtain:
This equation demonstrates that the slope of ln (Re−R(t)) against time is the water vapor isotopic exchange coefficient . Knowing λ, we can deduce the isotopic fractionation coefficient α for each isotope.
However, when the environmental humidity differs from the initial humidity inside the air bag, a numerical solution is required to solve the differential equation for R (Eq. 8).
2.2 Reconstructing initial water vapor isotopic compositions
The isotopic composition of the air bag water vapor undergoes an exponential evolution over time (Eq. 12). This method of applying exponential evolution equations to reconstruct isotopic compositions has been used in environmental forensics to investigate shifts in water isotopes due to metabolic changes, environmental conditions, or diet (Cerling et al., 2006; Ayliffe et al., 2004). Similarly, in climatology, this method helps retrieving initial isotopic composition from samples such as ice cores or tree rings, affected by evaporation, precipitation, and temperature fluctuations, facilitating historical climate reconstruction (Brienen et al., 2016). Here we apply a similar method, and apply the analytical solution of Eq. (8) using data from experiments in which the condition that q0 equals qe is met to determine the equation parameters.
The constants (λ, α18O, α2H) can be determined through laboratory experiments and Eqs. (10) and (13) (see Sects. 3.2 and 4.1). If we know the initial values within the air bag (q0, R18O0, R2H0), the ambient values (qe, R18Oe, R2He) during storage (approximated using ground-level conditions), and the storage time after sampling (Tstorage), we are able to simulate the variations in humidity and isotopic ratios inside the air bag according to Eqs. (5) and (8). Similarly, if we know Tstorage, the humidity and isotopic composition at time t = Tstorage (, R18O, R_2H) in the air bag, and the ambient values, we can deduce the initial values in the air bag at t = 0 by back-calculating. The equation used for reconstructing the initial isotope ratio (R0) is:
where R0 represents the initial isotopic ratio to be reconstructed, Rmeasured is the observed isotopic ratio after Tstorage, and is defined in Eq. (8).
This approach allows us to correct for diffusion-induced isotopic shifts and reconstruct the original vapor composition.
For mathematical clarity and consistency, isotopic ratios (R) are used in the equations presented in previous sections. Replacing R with δ-values would only shift the physical basis without affecting the mathematical validity of the equations or the estimation of α, as the standard ratio cancels out. For clearer visualization, δ-values are used for numerical applications and in the subsequent figures and tables.
The δ and d-excess values used in this study follow standard definitions:
Here, and , corresponding to the VSMOW international reference values.
3.1 Air bag isotope measurements
In this study, we used 0.5 and 4 L Teflon air bags produced by Dalian Hede Technologies Co., Ltd to collect and store vapor, and measured the vapor isotopes using a Picarro 2130i water isotope analyzer. Based on our testing and the airflow rate set for the Picarro analyzer, the 0.5 and 4 L bags provided sufficient sample volume for approximately 17 and 130 min of analysis, respectively.
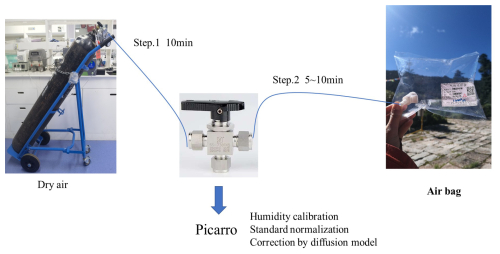
Figure 2Setup for isotope measurements using air bags with a Picarro atmospheric water vapor isotope analyzer.
Figure 2 illustrates the setup for measuring vapor isotopes in air bags. In this system, the air inlet of the Picarro isotope analyzer was connected to a three-way valve through Teflon or stainless-steel tubing. The other two ports of the three-way valve were attached to the outlet valve of the air bag and a dry air cylinder, respectively. Sample storage and measurement were conducted in a temperature-regulated room to maintain constant temperature conditions for the air bags and tubing.
In the measurement procedure, we first activated the dry air cylinder and adjusted the pressure reducing valve to 13.8 kPa (2 psi, pounds of force per square inch), within the Picarro water isotope analyzer's recommended range of 13.8–27.6 kPa (2–4 psi) for carrier gas. The instrument's built-in flow regulation maintains a gas flow rate of 40 sccm at 760 Torr, corresponding to ∼ 30–50 mL min−1 in our measurement conditions, ensuring stable sample delivery. We then opened the valve channel connecting the dry air cylinder to the three-way valve, allowing dry air to flow into the isotope analyzer and flush all air pathways for 10 min. Subsequently, we closed the dry air channel, opened the air bag outlet valve, and the corresponding valve channel on the three-way valve. This allowed water vapor in the air bags to be analyzed at a constant temperature in the Picarro analyzer, a process lasting between 5 and 10 min. Upon completion, we switched the valve to measure dry air. By repeatedly measuring isotopic composition for replicate samples – air samples collected simultaneously under the same conditions – we ensured the consistency of the water vapor measurements.
To correct isotope measurement bias caused by the instrument's sensitivity to different water vapor concentrations(Schmidt et al., 2010), we used the built-in Standard Delivery Module (SDM) of the Picarro L2130-i water vapor isotope analyzer to generate a 500–25 000 ppm water vapor gradient for isotope measurements. We selected 20 000 ppm as a reference humidity level, as this corresponds to the optimal accuracy range of the Picarro analyzer (Liu et al., 2014; Schmidt et al., 2010). All the measured vapor isotope data were normalized to the VSMOW-SLAP scale using two distinct laboratory reference waters with known isotopic composition. Before conducting daily measurements, we adjusted the quantity of the injected liquid standard to align with the humidity of the external vapor measurements.
3.2 Laboratory permeability experiments
To evaluate the variations of the diffusion of water molecules between the interior and exterior of the air bags, we conducted the following experiments (Table A1), as detailed below.
3.2.1 Experiment No. 1: Quantification of λsurface
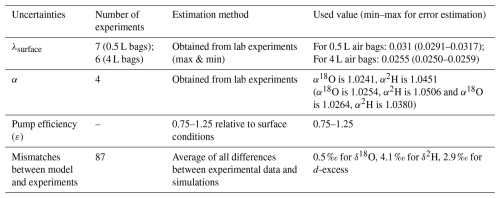
To quantify the water vapor exchange coefficient at the surface, λsurface, using Eq. (10), we filled the empty and clean air bags with dry air and measured humidity variations using a Picarro analyzer at intervals of 1 minute, 2, 4, 6, 8, and 10 h following the measurement method described in Sect. 3.1. As λ is related to the surface area of the sampling bag, measurements were conducted using 0.5 and 4 L air bags, with repetitions on both identical and different air bags of the same dimensions (refer to the experiment times in Table 1 and results in Fig. 3a). We measured λsurface () under near-surface pressure and temperature we set in a controlled chamber. While k may vary with temperature and pressure, these effects were assumed negligible within the condition of our study. Results will be shown in Sect. 4.1.
3.2.2 Experiment No. 2: Quantification of α
To investigate isotope variation patterns and improve measurement accuracy during storage in the air bag to quantify the isotopic fractionation coefficient α, using Eq. (13), initial values significantly different from ambient conditions were selected. Empty, clean air bags were first filled with dry air, then sealed by closing the bag valve. To maintain a closed system while injecting reference water, a dedicated injection septum was installed on the valve. After reopening the valve, a fixed amount of laboratory reference liquid water with known isotopic composition was injected into the dry air-filled bag using a 10 µL injection needle. In Experiment No. 2, we ensured that the initial humidity (q0) was approximately equal to the environmental humidity (qe). To ensure q0 = qe, the environmental vapor concentration was first measured, followed by the calculation and experimental determination of the water volume to be injected into the air bag. Water vapor concentration and isotope variations within the air bags were then measured using a Picarro analyzer at intervals of 5 min, 2, 4, 6, 8, and 10 h. Results will be shown in Sect. 4.1. To ensure data consistency and reliability, we repeated these measurements multiple times using air bags of both the same and different sizes, including 0.5 and 4 L bags. For the 0.5 L air bags, a separate bag was prepared for each time interval, and water vapor concentration and isotopic compositions were measured once to ensure that the parameter M remained stable without being affected by repeated measurements. However, manual injection of reference water with known isotopic composition introduced some variability, causing minor variations in initial values across all bags. This is evident in Figs. 5, 6, and A2, where data exhibit slight fluctuations due to the use of separate bags for each time interval. To address this, we repeated the experiment with 4 L air bags, measuring the same air bag at different time intervals, which ensured consistent initial conditions at t=0 but allowed M to change over time. When air bags differ only in size, the parameter λ associated with A and M varies, while the isotopic fractionation coefficient α is theoretically constant. Both approaches could contribute to uncertainties in the mismatches between the model and experimental results. Therefore, we incorporated the results from both the 0.5 and 4 L experiments into our uncertainty estimation of these mismatches, as detailed in Sect. 3.2.3 and 3.4.2.
3.2.3 Experiment No. 3: Diffusion model validation
To validate the diffusion model under diverse conditions and evaluate its uncertainties, we repeated Experiment No. 2, but injected different amounts of water with known isotopic composition to achieve a range of humidities from approximately to qe. Using the method described in Experiment No. 2, we injected 6 to 50 µL of reference water into a 4 L air bag filled with dry air to achieve the desired humidity range. Additionally, we repeated the experiment using two reference waters with distinct isotopic compositions, specifically δ18O = −58.07 ‰, δ2H = −447.41 ‰ and δ18O = −29.84 ‰, δ2H = −222.84 ‰. To assess extended-duration variations, we also lengthened the time interval to 24 h.
Once the parameters of the diffusion model have been obtained through Experiments No. 1 and 2, we used this model to simulate the variations in water vapor humidity and isotopic composition inside the air bag over time for Experiments No. 2 and 3 (refer to Sect. 2). When simulating these experiments using the diffusion model, we used measurements taken after a 5 min delay as the initial condition to ensure that it represented complete evaporation of the injected water. We then simulated the temporal variations in humidity and vapor isotopes within the air bag using a 5 min time step using Eqs. (5) and (8), separately. The resulting outputs (hereafter referred to as “the diffusion model simulations”) will be shown in Sect. 4.2 and code are available in the Code availability section.
3.3 Drone-mounted systems and field campaign
We designed and built a collection module for fixed-height sampling, incorporating diaphragm vacuum pumps, a rudder mounted on the drone, and a control module linked to a remote operating system. Before flight, each air bag was evacuated on the ground using the diaphragm pumps. When inactive, these self-sealing pumps effectively isolated the interior of the air bags from the external environment, minimizing diffusion prior to sampling. When the drone reaches a specified altitude, we remotely activate the designated air pump to inflate a specific air bag. Once sampling is complete, the pump is deactivated, and the drone ascends to the next target altitude, where the corresponding air pump inflates another air bag. This process was repeated until all predetermined samples were collected. After each sampling, the pump remained sealed, ensuring no unintended air ingress during descent. Additionally, due to the flexible nature of the air bags, internal and external pressures remained balanced. As air pressure increases during the drone's descent after collection. To further prevent the loss of collected air samples, a one-way valve was installed to block backflow. Additionally, the one-way valve helps prevent large droplets from entering the air bag during the collection process.
The sampling module was mounted on our specially designed high-altitude drones and deployed during a field campaign conducted from 25 June to 17 October 2020, in the pristine forests of Mountain Laojun, Lijiang, on the southeastern edge of the Tibetan Plateau and the northwestern of the Yunnan-Guizhou Plateau, China. We collected water vapor samples every 500 m, starting from near the surface along the vertical profile. To optimize sampling across different altitude ranges, we deployed UAVs designed for varying flight altitudes. Generally, the UAV operating at lower altitudes collected samples at seven heights from 4000 to 7000 m in a single flight. The mid-altitude UAV collected samples at four heights from 7500 to 9000 m in one flight, while the high-altitude UAV collected samples at four heights from 9500 to 11 000 m in two flights. Each flight took approximately 20–30 min. In case of any disruptions during sampling, we repeated the process until a complete vertical profile was obtained. At the beginning of the experiment, we also collected replicate samples at each height to ensure data consistency.
By integrating high-altitude drone sampling with subsequent water vapor isotope analysis using the Picarro analyzer at the surface, we obtained vapor isotopic profiles up to an altitude of 11 km. Unlike conventional methods, such as cryogenic vapor sampling, this approach requires much less sample volume and allows for timely measurements. Additionally, compared to large aircraft, airships, and balloon-based observations, this method is relatively low-cost and supports more flexible and long-term observations.
3.4 Application of diffusion model to vertical profiles
3.4.1 Estimating the air mass in the bag
λ is defined as and depends on the air mass M in the bag. In drone-based vertical sampling, M varies with altitude due to pressure changes, requiring an estimate of λ for different altitudes (λalt). However, since λ is an intrinsic property of the bag material, its apparent variation reflects uncertainties in estimating collected M, which depend on atmospheric pressure (P), sampling time, and pump efficiency (ε):
where Malt is the air mass collected at a different altitude and Msurface represents the air mass collected at the surface. At higher altitudes, where the air pressure (Palt) is lower than at the surface (Psurface), less air will be pumped into the air bag. To compensate for this effect, a longer sampling time was used to collect air at higher altitudes (Sampling timealt) than at the surface (Sampling timesurface) (Fig. A1).
Given that λalt is proportional to Malt, we calculated it as:
where λsurface is the λ quantified experimentally at the surface.
Since air pressure and sampling times were directly measured, the primary source of error for Malt, and consequently λalt, arises from pump efficiency (ε), which may decrease over time and at lower pressures. Using the estimated λalt, the observed vertical isotope profiles were corrected based on Eq. (14) from Sect. 2.2. The uncertainty estimation is discussed in Sect. 3.4.2.
3.4.2 Method of uncertainty estimation
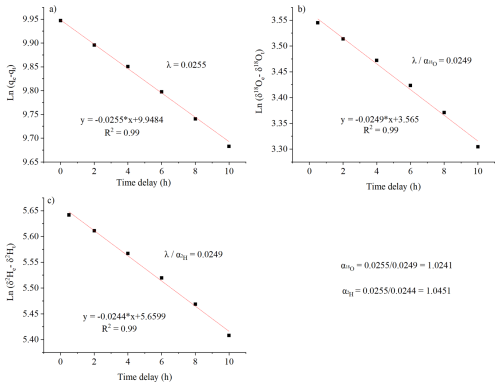
Potential errors in correcting vertical profiles using the diffusion model include estimates of λsurface, α, pump efficiency (ε), and mismatches between model and experiments (Table 1). We detail each below:
-
λsurface uncertainty: laboratory experiments provided upper and lower bounds on λsurface (Sect. 4.1). These were used for error estimation.
-
α uncertainty: the λ and were first estimated from several experiments, from which α was calculated. Their averaged values were used separately to parameterize the model. As highlighted in Sect. 3.2.2, estimating (and subsequently calculating α) requires results from cases where qe equals qe, minor variations in q0 and fluctuations in qe could introduce non-systematic discrepancies between the model and experimental results. Consequently, for analyzing the contribution of α to uncertainties, only α derived from experiments where the model closely matched the majority of experimental results were considered. Selection criteria for these experiments included minimal deviation between q0 and qe, minimal deviation between experimental data and simulations, and stable qe, ensuring the reliability of the chosen α.
-
Pump efficiency (ε) uncertainty: The efficiency of the pump may decline over time or vary with atmospheric pressure, affecting the collected air mass M. To account for this, we applied a conservative uncertainty range of 0.75 to 1.25 relative to surface conditions, ensuring the full range of possible variations in Malt was considered.
-
Model-experiment mismatches: We compared model simulations with experimental data across 87 cases, calculating the average absolute discrepancy. These mismatches were included as an additional uncertainty component.
Total Uncertainty Calculation: The maximum discrepancy across all calibration results – using the full uncertainty range for λsurface, α, and pump efficiency (ε) – was determined. The model-experiment mismatch was then added as an independent error component. The final uncertainty estimates, reported in Sect. 4.3 to 4.5, account for all potential error.
3.5 Satellite isotope data from IASI
Several satellite missions have contributed to water vapor isotope observations, including the Tropospheric Emission Spectrometer (TES) onboard Aura (2004–2018) (Worden et al., 2006), the Scanning Imaging Absorption Spectrometer for Atmospheric Cartography (SCIAMACHY) onboard Envisat (2002–2012), the Atmospheric Infrared Sounder (AIRS) onboard Aqua (since 2002) (Worden et al., 2019), and the Tropospheric Monitoring Instrument (TROPOMI) onboard Sentinel 5 Precursor (since 2017) (Schneider et al., 2020). In this study, we use the MUSICA retrievals from the Infrared Atmospheric Sounding Interferometer (IASI) onboard METOP due to its broad spatiotemporal coverage, vertical profiling capability, and the availability and accessibility of its dataset (Diekmann et al., 2021). It has a horizontal footprint of approximately 12 km at nadir (directly below the satellite), increasing with the angle of observation. This configuration ensures nearly global coverage twice daily.
In this study, as no satellite retrievals of δ18O are currently available, we compared our observed vapor δ2H profiles up to the upper troposphere with satellite observations. Due to the intermittent availability of IASI data at any given location, we limited our comparison of observational results across various altitudes at our study site to days when IASI data were available. Satellite measurements, particularly for vertical profiles of water vapor isotopes, are inherently different from direct sampling, they represent a vertical average over layers determined by the averaging kernels (Rodgers and Connor, 2003; Worden et al., 2006). Therefore, their comparability with ground-based or drone-based observations, which provide high-resolution local data, is limited. While using averaging kernels to smooth the observed profile could facilitate a more quantitative analysis, we simply averaged the observations for the corresponding altitudes. Consequently, the comparison remains mainly qualitative.
4.1 Parameter estimates
The λsurface was determined through laboratory Experiment No. 1. Based on Eq. (10), the parameter λsurface was estimated to be 0.0312 (uncertainty range: 0.0291 to 0.0317) for the 0.5 L air bags and 0.0255 (uncertainty range: 0.0250 to 0.0259) for the 4 L air bags (Fig. 3a, Table 1).
The isotopic fractionation coefficients α, were determined through laboratory Experiment No. 2. From the averaged results of these measurements, using Eq. (13), the water vapor isotopic exchange coefficient, expressed as , was estimated to be 0.0249 for 18O and 0.0244 for 2H (Fig. 3b and c). Consequently, α18O was estimated to be 1.0241 (0.0255/0.0249), and α2H was estimated to be 1.0451 (0.0255/0.0244) (Fig. 3b and d). Two additional sets of fractionation coefficients α, were obtained: α18O = 1.0254, α2H = 1.0506, and α18O = 1.0264, α2H = 1.0380 (Table 1).
The specific parameter values obtained in this study pertain to the Teflon air bags used in the aforementioned tests, conducted at an ambient temperature of 16 °C. These values depend on bag material, temperature, and pressure, which should be considered when applying the model under different conditions. We also noted batch-to-batch variations among air bags from the same manufacturer. We apply α measured under ground-level storage and measurement conditions, assuming negligible temperature and pressure effects during the short (10–20 min) drone-based sampling period. Future work is needed to quantify these dependencies.
4.2 Diffusion model validation
4.2.1 General case
To validate the model, we used Experiment No. 3 described in Sect. 3.2. The diffusion model simulations (lines in Figs. 4, 5, 6, and A2) are in close agreement with our experimental observations (markers in Figs. 4, 5, 6, and A2), showing consistency in humidity, δ18O, δ2H, and d-excess variations, with only minor deviations. Shorter storage times produce fewer deviations. When the humidity inside the air bag is lower than the ambient level, vapor from the environment enters the air bag, resulting in a gradual increase in humidity (Figs. 4a and A2). Meanwhile, because the water vapor isotopic composition in the air bag is significantly lower than the ambient values, δ18O and δ2H in the air bag gradually increase and toward ambient values as ambient moisture enters the bags over time (Fig. 4b and c). In contrast, the d-excess increase as the time delay progresses due to kinetic fractionation during moisture diffusion into the air bag (Fig. 4d), as detailed in the following Sect. 4.2.2.
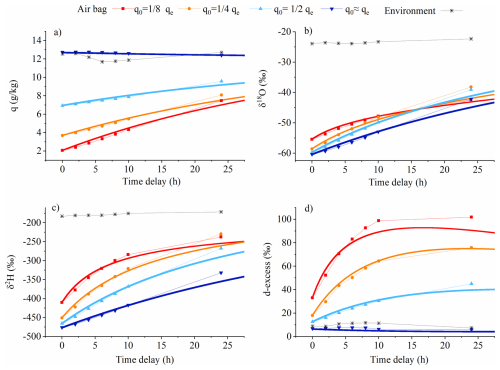
Figure 4Comparison of the variation within the air bag over time in laboratory permeability experiments (markers) and diffusion model simulations (lines) across a range of initial humidities at t = 0 (q0), ranging from approximately to qe (qe denotes the environmental humidity), under the condition that the water vapor isotopic composition in the air bag significantly differs from ambient values: (a) humidity; (b) δ18O; (c) δ2H, (d) d-excess.
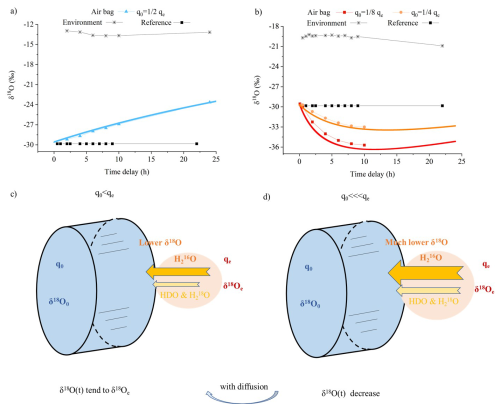
Figure 5(a–b) Variations of δ18O under different conditions: (a) when both the differences between internal (δ18O0) and external (δ18Oe) δ18O as well as between internal (q0) and external (qe) humidity are not significant, δ18O gradually increases toward equilibrium; (b) when q0 is significantly lower than qe, a stronger vapor influx causes enhanced kinetic fractionation, leading to a decrease in δ18O. (c–d) Corresponding schematics: panel (c) illustrates the mechanism for panel (a), where a weaker humidity gradient results in slower isotopic shifts, while panel (d) corresponds to panel (b), showing intensified fractionation with a larger gradient, with arrows indicating vapor flux direction and fractionation intensity. δ18O (t) is the variation of δ18O within the air bag over time. In panels (a) and (b), the colored lines represent model simulations based on Eq. (8), using parameterization from Experiments No. 1 and 2. Colored square markers show the corresponding experimental observations within the air bags. Black square markers indicate the initial values inside the air bag, which remain constant over time and serve as a reference for comparison (legend: Reference).
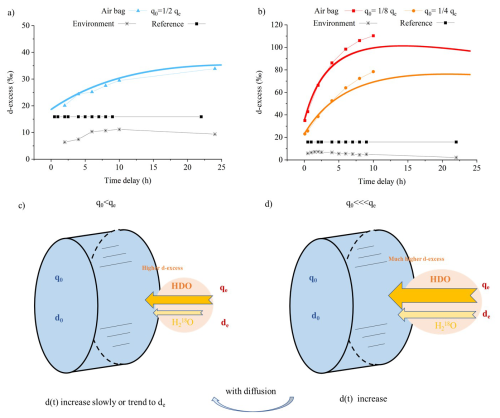
Figure 6(a–b) Same as Fig. 5, but showing the evolution of d-excess: (a) when the difference between the humidity inside (q0) and outside (qe) the air bag is not significant, d-excess increases gradually; (b) when q0 is significantly lower than qe, a stronger vapor influx enhances kinetic fractionation, causing a more rapid d-excess increase. (c–d) Corresponding schematics: panel (c) illustrates the mechanism for panel (a), where a smaller humidity gradient results in slower isotopic shifts, while panel (d) corresponds to panel (b), showing intensified fractionation with a larger gradient. d0 indicates the initial d-excess at t = 0, de represents the d-excess in the environment. d(t) denotes the variation of d-excess within the air bag over time.
We observed that some curves unexpectedly intersect, which can be understood by analyzing Eq. (8). The first term continuously drives R(t) towards Re, while the second term modulates the rate of change. Initially, for simulations with lower q0 (e.g., the red and orange curves), qe−q(t) is large, making the second term significant and positive, thereby increasing R(t) more rapidly. However, as R(t) exceeds , the sign of this term reverses, slowing down the increase in R(t) compared to other curves. In contrast, curves with higher initial q0 (e.g., blue curve) experience a steadier growth and eventually surpass the initially faster-growing curves, leading to the observed crossing.
4.2.2 Particular cases
Due to isotopic kinetic fractionation, lighter HO molecules preferentially diffuse into the air bag compared to heavier isotopologues such as HD16O and HO, resulting in a vapor flux with lower δ18O relative to ambient vapor (Fig. 2). Moreover, variations within the air bag are driven by differences in water vapor content and isotopic ratios between its interior and exterior, as described in the diffusion model in Sect. 2. As shown in the initial experiment (Fig. 4b and c), when the internal δ18O and δ2H are significantly below the ambient values, the δ-values of the diffusing vapor, although lower than ambient, still exceeds the initial internal δ-values, leading to a gradual increase towards the ambient values; diffusion simply equilibrates the isotopic composition in the bag and the environment. When the disparity between internal and external δ18O or δ2H is not very substantial, and humidity differences are also minimal, the weaker diffusive gradient produces less net kinetic fractionation. This results in a small amount of vapor with lower δ18O and δ2H than the ambient moisture entering preferentially, but not falling below internal initial values, thereby still drives a progressive increase in the internal values towards ambient moisture (Fig. 5a and c). In contrast, with the same initial contrast between internal and ambient δ-values, that is, the disparity between internal and external δ18O and δ2H is less pronounced, but qe is much lower than qe, there is a stronger net flux into the bag, and this flux fractionates more rapidly; much more vapor with significantly lower δ18O and δ2H than the ambient moisture (and lower values than the initial internal vapor) enters the air bags and dominates their isotopic composition, thereby reducing the internal δ-values (Fig. 5b and d). The smaller the difference in humidity and isotopic composition between the inside and outside of the air bag, the slower and smaller the isotopic change in the vapor within the air bag (Figs. 5 and 6).
In addition, because HDO and HO diffuse at similar rates the magnitude of the kinetic fractionation for D and 18O is similar. However, since d-excess reflects deviations relative to the 8 : 1 fractionation ratio typical of equilibrium processes, the tendency is for kinetic fractionation during diffusion to contribute vapor with high d-excess and cause an increase in d-excess during air bag storage as water vapor is added to the bag (Fig. 6a and c). When the humidity difference between the inside and outside of the air bag increases, the d-excess of the incoming vapor flux increases as a result of more intensive kinetic fractionation. This leads to a faster increase in the vapor d-excess inside the air bag (Fig. 6b and d).
Regardless of the differences in water vapor humidity and isotopic compositions inside and outside the air bag, the diffusion model simulations closely match the experimental observations (Figs. 4, 5 and 6). Using the method described in Sect. 3.4.2, the average difference between all simulations and experimental data for each parameter represented the model-experiment mismatch: 0.5 ‰ for δ18O, 4.1 ‰ for δ2H, and 2.9 ‰ for d-excess.
4.3 Raw and corrected vertical profiles
Here, we present a summary of drone-based observations from the field campaign at Mount Laojun, Lijiang, on the southeastern edge of the Tibetan Plateau and the northwestern of the Yunnan-Guizhou Plateau, China, conducted between 25 June and 17 October 2020.
As altitude increases, vapor δ18O and δ2H decrease due to condensation and precipitation processes that occur as air masses ascend, which preferentially remove heavier isotopes following Rayleigh distillation (Dansgaard, 1964). Meanwhile, the d-excess rises. This pattern aligns with previous observations in the lower troposphere (Salmon et al., 2019; He and Smith, 1999) and simulations of complete vertical profiles (Bony et al., 2008).
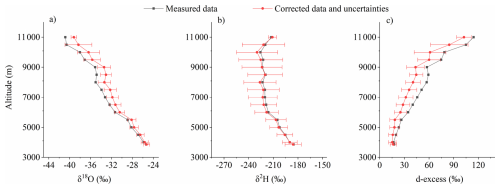
Figure 7Comparison of vertical profiles for the mean values of all raw measurements and corrected data from June to October, and associated uncertainties for δ18O (a), δ2H (b) and d-excess (c).
In our observations, the variation in δ18O across the vertical profile from ground level at 3856 m to 11 km is approximately 10 ‰–15 ‰. However, as altitude increases, the air becomes progressively drier, leading to a greater disparity in humidity between the air collected in the air bag and the surface storage environment. This aligns with the variations in Sect. 4.2.2 (Figs. 5b, d and 6b, d). The strong kinetic fractionation driven by the diffusion of air into the air bag results in a decrease in the water vapor δ18O within the bag. After applying model corrections, the corrected δ18O inside the bag increased slightly compared to pre-correction levels. As described in Sect. 4.2, vapor flux with higher d-excess entering the bag increases the d-excess inside. As a compensation, the diffusion model applies corrections, resulting in a reduced d-excess after correction (Figs. 7c and 10).
4.4 Uncertainty estimates
According to the method of uncertainty estimation elaborated in Sect. 3.4.2, we investigated four error sources to evaluate uncertainty (Table 1).
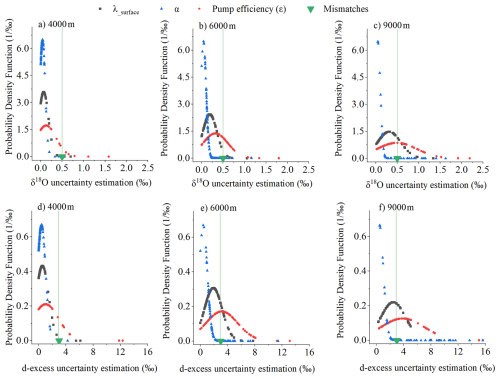
Figure 8Contributions of different sources of uncertainty for δ18O (a, b, c for 4000, 6000, 9000 m, respectively) and d-excess (d, e, f for 4000, 6000, 9000 m, respectively). The green triangle shows the average mismatch between the model and experimental results. The peak position of each probability density function (PDF) represents the most likely uncertainty value for a given source, while the width of the distribution reflects the variability.
We represent the contribution of each source of uncertainty to vertical vapor δ18O and d-excess measurements at different altitudes using probability density function plots (Fig. 8). Each PDF peak indicates the most probable uncertainty value, while its width reflects sensitivity to that parameter. Narrow PDFs suggest stable, well-constrained uncertainty sources, whereas broader PDFs indicate greater variability and a more diffuse impact on the correction outcome. The mismatch between the model and the experiment (green marker), calculated using the method described in Sect. 3.4.2, is assumed to remain constant across all altitudes. The other three error sources manifest as unimodal normal distributions at various altitudes for both δ18O and d-excess. Errors due to uncertainties in pump efficiency (ε) are the main source, exhibiting the largest spread (Fig. 8) and increasing with altitude (Fig. 9). Errors derived from λsurface and α also increase with altitude (Figs. 8 and 9). As a result, at higher elevations, the satellite δ2H differs more from the measured and corrected data than at lower altitudes (Fig. 10). This pattern arises because λalt deviates more from λsurface at higher elevations (Eq. 16), primarily due to increased errors in estimating Malt, amplifying correction errors. Moreover, the humidity and isotopic disparity between the air captured in the air bag and lower-altitude ambient air widens with altitude, requiring more intensive corrections. Consequently, both the uncertainty (Figs. 8 and 9) and the magnitude of the diffusion correction (Fig. 7) increase with altitude.
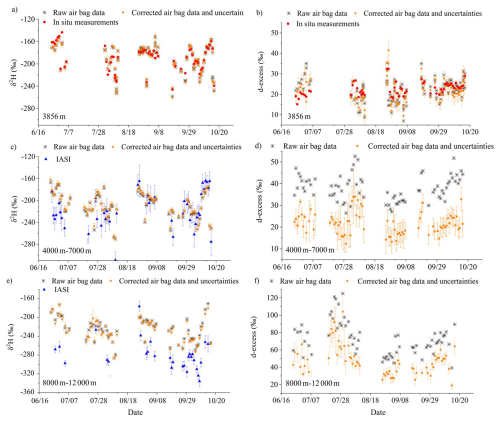
Figure 10Time series comparison for δ2H (a, c, e) and d-excess (b, d, f): (a, b) Raw and corrected (with uncertainties) instantaneous air bag measurements at the surface (3856 m), compared with Picarro in-situ measurements at the same altitude, averaged over the sampling period. (c, d) Raw and corrected (with uncertainties) altitude-averaged air bag measurements from 4000 to 7000 m, compared with satellite data (IASI), which also include uncertainty ranges accounting only for retrieval-fit noise and the atmospheric temperature a priori constraint. (e, f) Raw and corrected (with uncertainties) altitude-averaged air bag measurements from 8000 to 12000 m, compared with satellite data (IASI).
On the contrary, errors pertaining to λsurface and α diminish at altitudes exceeding 10 500 m. This phenomenon may be attributed to our sampling methodology. Although we collected air samples in sequence from low to high altitudes, these samples were measured in reverse order, from high to low altitudes. Consequently, samples taken from the highest altitudes had the shortest storage durations, typically ranging from 10 min to 2 h. Additionally, we extended the sampling times with increasing altitude (Fig. A1). These extended sampling periods and reduced storage durations help to partially offset the amplified disparities observed between the raw and corrected profiles at higher altitudes. During the drone-based sampling period, which lasted around 10-20 min, the external conditions impacting the air bags were not the ground-level environmental values used in the model but rather those of the vertical profile. However, the humidity at higher altitudes is lower than at ground level, and the isotopic composition are closer to those inside the airbag. Consequently, its impact on the airbag's internal conditions is less significant than suggested by using ground-level environmental values. Therefore, the overestimated error in our model accounted for these potential discrepancies.
The combined uncertainty from all sources, including λsurface, α, pump efficiency (ε), and model-experiment mismatches, result in a total uncertainty of approximately 1 ‰ for δ18O and 8 ‰ for d-excess across 98 % of the data. The corresponding 95 % confidence intervals are approximately 0.9 ‰ for δ18O and 6.9 ‰ for d-excess. This range is acceptable when compared to the substantial vertical variations we observed (∼ 20 ‰ for δ18O and ∼ 100 ‰ for d-excess). Among these sources, ε contributes the largest uncertainty, particularly at higher altitudes (Fig. 8), likely due to the conservative uncertainty range we applied to account for potential reductions in collected air mass at high altitudes. Additionally, λsurface and α also contributes considerably to the total uncertainty (Figs. 8 and 9). To mitigate this, we recommend conducting multiple measurements to obtain an averaged value and performing repeated parameter validation to ensure robustness.
4.5 Comparison with Picarro measurements and satellite data
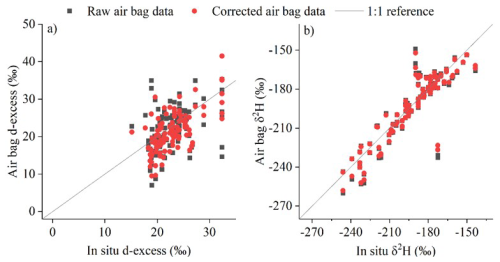
The left panels of Fig. 10 (Fig. 10a, c, and e) show the comparison of raw and corrected water vapor δ2H measurements at different altitudes with in-situ surface-level measurements on the Picarro or IASI satellite data at corresponding altitudes.
At near-ground level, both the raw and corrected δ2H and d-excess values from the bag measurements show close agreement with the in-situ Picarro observations (Fig. 10a and b), indicating minimal diffusion effects under nearly identical humidity and isotopic conditions between the inside and outside of the bags. Most data points are distributed along the 1 : 1 line (Fig. A3), while d-excess shows slightly larger scatter. After applying the correction, the agreement improved: the correlation coefficient between the bag and Picarro δ2H values increased from 0.90 to 0.99, and for d-excess from 0.41 to 0.72. The mean absolute error (MAE) between the corrected bag and Picarro measurements is 3.0 ‰ for δ2H and 1.4 ‰ for d-excess. These discrepancies are within the uncertainty range derived from laboratory diffusion experiments and model–experiment mismatches, which were comprehensively incorporated in the error estimation (Sect. 3.2 and 3.4): 0.5 ‰ for δ18O, 4.1 ‰ for δ2H, and 2.9 ‰ for d-excess. The differences also can be attributed to the fact that the Picarro in-situ observations are period-averaged, whereas the bag samples represent instantaneous values.
To compare corrected measurements with observations at higher altitudes, we refer to the IASI satellite dataset. We acknowledge that this comparison is qualitative, as satellite data represent vertical averages over layers defined by averaging kernels, and therefore differ in measurement footprints (both horizontal and vertical) and spatio-temporal sampling disparities (Shi et al., 2020). A more quantitative analysis could be facilitated if an averaging kernel is used to smooth the observed profiles (Herman et al., 2014), but even after such adjustment, substantial differences would likely persist. IASI provides only two overpasses per day (around 09:30 LT am and pm), whereas our drone-based measurements were conducted locally during daytime. The IASI footprint (∼ 12 km at nadir) provides coarse spatial averaging, whereas our measurements are local and instantaneous, limiting its ability to capture fine-scale variability at the sampling site. Importantly, IASI cannot observe through or below thick clouds such as anvils, and thus mainly samples air outside convective detrainment regions. Because convective detrainment isotopically enriches vapor (Kuang et al., 2003; Moyer et al., 1996; de Vries et al., 2022; Webster and Heymsfield, 2003; Smith et al., 2006), the air masses observed by IASI – being farther from detrainment – are expected to be more depleted. This cloud-sampling limitation may therefore contribute to the lower δ2H values retrieved by IASI in the upper troposphere compared with our in-situ observations. Such fundamental mismatches in vertical weighting, temporal coverage, and spatial representativeness are noted in previous satellite – in-situ intercomparisons, which found that satellites capture large-scale isotopic gradients but smooth out fine-scale vertical and diurnal variations (Lacour et al., 2018; Herman et al., 2014). These complementary characteristics highlight the value of combining satellite and high-resolution in-situ observations: satellites provide synoptic coverage, while local observations resolve small-scale processes that shape the isotopic structure of the atmosphere. Overall, our comparison with IASI should thus be regarded as a qualitative consistency check confirming large-scale and temporal coherence rather than a one-to-one correspondence.
For most intervals, IASI satellite data closely match raw and corrected δ2H measurements for altitudes 4000–7000 m. In the 8000–12000 m range, although applying the diffusion correction increased the correlation coefficient between the air-bag observations and the IASI data from 0.53 to 0.60, IASI data are lower than δ2H air-bag observations during certain periods, particularly June and September 2020; one of the possible reasons could be unaccounted environmental influences at high altitudes. However, it is noteworthy that the IASI data closely match the observed δ2H for all other periods in the 4000–7000 m range, except for June 2020, where they are lower too. Moreover, diffusion within the sampling bags could, in principle, introduce systematic bias, yet the IASI values sometimes agree well, sometimes appear higher, and sometimes lower than our measurements (Fig. 10). Therefore, the differences between our in-situ observations and the IASI retrievals likely arise mainly from representativeness differences intrinsic to the satellite data, as discussed above – practically the cloud-sampling limitation in the upper troposphere – rather than from diffusion-related biases. Accordingly, such variations cannot be corrected by the diffusion model, which accounts for isotopic fractionation during air-bag storage but not for spatiotemporal or cloud-sampling discrepancies between observation platforms.
The right panel of Fig. 10 (Fig. 10b, d, and f) shows the comparison of raw and corrected vapor d-excess measurements. No d-excess dataset is available for comparison for the 4000–7000 and 8000–12 000 m (Fig. 10d and f). As previously noted, corrected d-excess values should be and are lower than the raw data, as expected from diffusion theory. For the 8000–12 000 m observations, the correction magnitude is relatively smaller than at lower altitudes due to the shorter storage time of the air bags.
Although no completely independent dataset is available for direct validation, our model – already verified by laboratory experiments – was used to perform a detailed assessment of all potential error sources and to quantify a comprehensive and conservative uncertainty range of the corrected data.
High spatial and temporal resolution water vapor isotope data are critical for understanding various hydrologic cycle processes. However, observations of vertical water vapor isotope profiles are scarce, particularly in the upper troposphere. Satellite-derived vapor isotope data are available only at limited vertical and temporal resolutions. Acquiring high-resolution water vapor isotope data, especially under conditions where direct measurements are difficult, has been a significant challenge for the water isotopes research community. This study demonstrates the potential of a drone-based air bag sampling method to overcome this challenge, offers solutions for evaluating air bag suitability and addressing air bag permeability.
While air bags offer the advantage of sample collection, their inherent permeability can affect the sealing integrity of the samples, leading to potential contamination. The permeability of airbag materials varies, with some exhibiting lower levels. We recommend prioritizing the use of glass containers and air bags with the lowest permeability for collecting water vapor using portable devices. Additionally, it is essential to conduct the permeability experiments described in this article before any experimental undertaking. This involves storing water vapor with known isotopic composition in the portable collection device for an extended period and then re-measuring these values to assess or determine the device's permeability parameters.
To further address the permeability challenge, we developed a mathematical model to evaluate and correct for diffusion and isotopic fractionation, ensuring the reliability of vapor isotope measurements using air bags. Calibrated with parameters from laboratory experiments, our correction model reconstructs the initial isotopic composition of sampled vapor by using data from both the air bag and the surrounding environment, offers a potential solution to the prevalent permeability challenges. This model was rigorously validated against observational experiments conducted under varying conditions. We also applied this model to drone-collected samples at various pressures. By estimating uncertainty and comparing corrected data with in-situ surface-level measurements on the Picarro and satellite observations, we validated the reliability and applicability of drone-based water vapor isotope measurements.
Our drone-based sampling system, combined with the diffusion model, effectively addresses the limitations of traditional high-altitude water vapor measurement methods. It meets the need for lightweight equipment while providing a more economical, efficient, and flexible alternative to conventional approaches involving large aircraft, airships, and balloons. This approach enables us to exploit the benefits of drone-based air bag sampling at high altitudes and over long distances, while effectively mitigating its potential limitations. This strategy significantly broadens its potential applications across various environments, thereby enhancing the range and richness of data that can be gathered for water vapor isotope research.
This study was conducted under stable temperature and equal internal and external pressures during storage. We acknowledge that a formulation based on partial pressure of water vapor would be more general and could improve model applicability under varying temperature and pressure conditions. Future work could extend the model to account for these factors.
Table A1Summary of experiments: diffusion parameter quantification, model validation and differences in experimental methods. λsurface denotes the water vapor exchange coefficient at the surface, α refers to the fractionation coefficient of isotopes, q0 represents the initial vapor humidity in the air bags, and qe corresponds to the environmental vapor humidity.
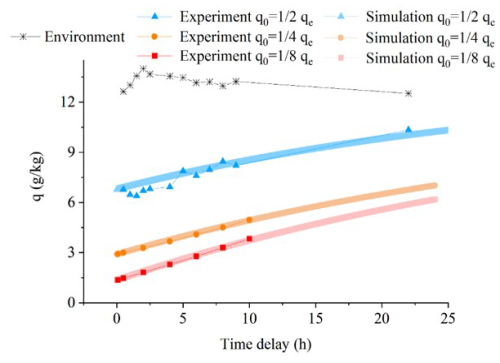
Figure A2Comparison of humidity variation within the air bag over time in laboratory permeability Experiments No. 3 (markers) and diffusion model simulations (lines) for Figs. 4 and 5. The experiments cover a range of initial humidities at t = 0 (q0), from approximately to , where qe denotes environmental humidity.
The diffusion modeling code, which simulates the evolution of water vapor isotopes in the airbag and corrects them to their initial values, is available at: https://doi.org/10.5281/zenodo.17790553.
Data are available from the authors on request.
A video showcasing our field campaign on drone-derived water vapor isotope sampling up to the upper troposphere (11 km) during convective activity, including the workflow for airbag water vapor isotope sampling, is currently available upon request and will be publicly accessible in the near future. Please contact the corresponding author for access (di.wang@latmos.ipsl.fr).
D.W., C.R., and L.T. designed the research, established the subjects of the methodology, and performed the analysis; D.W. and D.Y. conducted the observations; G.B., S.F., H.P. and L.L. contributed to the establishment of methodologies, data calibration, and analysis; Y.S. provided assistance with the computing; All authors contributed to the discussion of the results and the final article; D.W. drafted the manuscript with contributions from all co-authors.
The contact author has declared that none of the authors has any competing interests.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors. Views expressed in the text are those of the authors and do not necessarily reflect the views of the publisher.
The authors gratefully acknowledge the Infrared Atmospheric Sounding Interferometer (IASI) for providing spatiotemporal coverage of δ2H retrieval. We thank Jiaping Xu, Mingjian Chen, Le Chan, and Yao Yao for their irreplaceable help in the development of the drones. We are grateful to the management of 99 Dragon, Mountain Laojun in Lijiang for facilitating the field experiments. We thank Pengbin Liang and Jiangrong Tai for partially participating in the field observations. We acknowledge Hans Christian Steen-Larsen and Harald Sodemann for their discussions on the correction method. We acknowledge the use of OpenAI's ChatGPT to assist in refining the language. This work was supported by the National Natural Science Foundation of China (Grants Nos. 42401043, 42271143 and U2202208), the fellowship from the China Postdoctoral Science Foundation (Grant No.2025T180092). Di Wang acknowledges support from Chinese Scholarship Council and the Postdoctoral Fellowship Program of China Postdoctoral Science Foundation (Grant No. GZC20241440). Siteng Fan acknowledges support from the Marie Skłodowska-Curie Actions of European Research Executive Agency (Grant No. 101064814) and the High Level Special Fund of Southern University of Science and Technology (Grant No. G03050K001).
This research has been supported by the National Natural Science Foundation of China (grant nos. 42401043, U2202208 and 42271143), the fellowship from the China Postdoctoral Science Foundation (grant nos. 2025T180092 and GZC20241440), the Marie Skłodowska-Curie Actions of European Research Executive Agency (grant no. 101064814), the High Level Special Fund of Southern University of Science and Technology (grant no. G03050K001).
This paper was edited by Christof Janssen and reviewed by Bibhasvata Dasgupta and two anonymous referees.
Ayliffe, L., Cerling, T., Robinson, T., West, A., Sponheimer, M., Passey, B., Hammer, J., Roeder, B., Dearing, M., and Ehleringer, J.: Turnover of carbon isotopes in tail hair and breath CO2 of horses fed an isotopically varied diet, Oecologia, 139, 11–22, https://doi.org/10.1007/s00442-003-1479-x, 2004.
Bony, S., Risi, C., and Vimeux, F.: Influence of convective processes on the isotopic composition (δ18O and δD) of precipitation and water vapor in the tropics: 1. Radiative-convective equilibrium and Tropical Ocean–Global Atmosphere–Coupled Ocean-Atmosphere Response Experiment (TOGA-COARE) simulations, Journal of Geophysical Research: Atmospheres, 113, https://doi.org/10.1029/2008jd009942, 2008.
Bowen, G. J., Cai, Z., Fiorella, R. P., and Putman, A. L.: Isotopes in the Water Cycle: Regional-to Global-Scale Patterns and Applications, Annual Review of Earth and Planetary Sciences, 47, https://doi.org/10.1146/annurev-earth-053018-060220, 2019.
Brienen, R. J., Schöngart, J., and Zuidema, P. A.: Tree rings in the tropics: insights into the ecology and climate sensitivity of tropical trees, Tropical tree physiology: adaptations and responses in a changing environment, 439–461, https://doi.org/10.1007/978-3-319-27422-5_20, 2016.
Cerling, T. E., Wittemyer, G., Rasmussen, H. B., Vollrath, F., Cerling, C. E., Robinson, T. J., and Douglas-Hamilton, I.: Stable isotopes in elephant hair document migration patterns and diet changes, Proceedings of the National Academy of Sciences, 103, 371–373, https://doi.org/10.3410/f.1030596.358704, 2006.
Dansgaard, W.: Stable isotopes in precipitation, Tellus, 16, 436–468, https://doi.org/10.1111/j.2153-3490.1964.tb00181.x, 1964.
de Vries, A. J., Aemisegger, F., Pfahl, S., and Wernli, H.: Stable water isotope signals in tropical ice clouds in the West African monsoon simulated with a regional convection-permitting model, Atmos. Chem. Phys., 22, 8863–8895, https://doi.org/10.5194/acp-22-8863-2022, 2022.
Diekmann, C. J., Schneider, M., Ertl, B., Hase, F., García, O., Khosrawi, F., Sepúlveda, E., Knippertz, P., and Braesicke, P.: The global and multi-annual MUSICA IASI {H2O,δD} pair dataset, Earth Syst. Sci. Data, 13, 5273–5292, https://doi.org/10.5194/essd-13-5273-2021, 2021.
Fick, A.: Ueber diffusion, Annalen der Physik, 170, 59–86, 1855.
Galewsky, J., Steen-Larsen, H. C., Field, R. D., Worden, J., Risi, C., and Schneider, M.: Stable isotopes in atmospheric water vapor and applications to the hydrologic cycle, Reviews of Geophysics, 54, https://doi.org/10.1002/2015rg000512, 2016.
Gat, J. R.: Oxygen and hydrogen isotopes in the hydrologic cycle, Annual Review of Earth and Planetary Sciences, 24, 225–262, https://doi.org/10.1146/annurev.earth.24.1.225, 1996.
Ghosh, P. and Brand, W. A.: Stable isotope ratio mass spectrometry in global climate change research, International Journal of Mass Spectrometry, 228, 1–33, https://doi.org/10.1016/s1387-3806(03)00289-6, 2003.
Gralher, B., Herbstritt, B., and Weiler, M.: Technical note: Unresolved aspects of the direct vapor equilibration method for stable isotope analysis (δ18O, δ2H) of matrix-bound water: unifying protocols through empirical and mathematical scrutiny, Hydrol. Earth Syst. Sci., 25, 5219–5235, https://doi.org/10.5194/hess-25-5219-2021, 2021.
Grootes, P. M. and Stuiver, M.: Oxygen 18/16 variability in Greenland snow and ice with 10(-3)- to 10(5)-year time resolution, Journal of Geophysical Research-Oceans, 102, 26455–26470, https://doi.org/10.1029/97jc00880, 1997.
Havranek, R. E., Snell, K. E., Davidheiser-Kroll, B., Bowen, G. J., and Vaughn, B.: The Soil Water Isotope Storage System (SWISS): An integrated soil water vapor sampling and multiport storage system for stable isotope geochemistry, Rapid Communications in Mass Spectrometry, 34, e8783, https://doi.org/10.1002/rcm.8783 , 2020.
He, H. and Smith, R. B.: Stable isotope composition of water vapor in the atmospheric boundary layer above the forests of New England, Journal of Geophysical Research Atmospheres, 104, 11657–11673, https://doi.org/10.1029/1999jd900080, 1999.
Hendry, M. J., Schmeling, E., Wassenaar, L. I., Barbour, S. L., and Pratt, D.: Determining the stable isotope composition of pore water from saturated and unsaturated zone core: improvements to the direct vapour equilibration laser spectrometry method, Hydrol. Earth Syst. Sci., 19, 4427–4440, https://doi.org/10.5194/hess-19-4427-2015, 2015.
Herbstritt, B., Gralher, B., Seeger, S., Rinderer, M., and Weiler, M.: Technical note: Discrete in situ vapor sampling for subsequent lab-based water stable isotope analysis, Hydrol. Earth Syst. Sci., 27, 3701–3718, https://doi.org/10.5194/hess-27-3701-2023, 2023.
Herman, R. L., Cherry, J. E., Young, J., Welker, J. M., Noone, D., Kulawik, S. S., and Worden, J.: Aircraft validation of Aura Tropospheric Emission Spectrometer retrievals of HDO H2O, Atmos. Meas. Tech., 7, 3127–3138, https://doi.org/10.5194/amt-7-3127-2014, 2014.
Hodges, J. T. and Lisak, D.: Frequency-stabilized cavity ring-down spectrometer for high-sensitivity measurements of water vapor concentration, Applied Physics B, 85, 375–382, https://doi.org/10.1007/s00340-006-2411-y, 2006.
Johnson, L., Sharp, Z., Galewsky, J., Strong, M., Van Pelt, A., Dong, F., and Noone, D.: Hydrogen isotope correction for laser instrument measurement bias at low water vapor concentration using conventional isotope analyses: application to measurements from Mauna Loa Observatory, Hawaii, Rapid Communications in Mass Spectrometry, 25, 608–616, https://doi.org/10.1002/rcm.4894, 2011.
Kuang, Z., Toon, G. C., Wennberg, P. O., and Yung, Y. L.: Measured HDO/H2O ratios across the tropical tropopause, Geophysical Research Letters, 30, https://doi.org/10.1029/2003gl017023, 2003.
Lacour, J.-L., Risi, C., Worden, J., Clerbaux, C., and Coheur, P.-F.: Importance of depth and intensity of convection on the isotopic composition of water vapor as seen from IASI and TES δD observations, Earth and Planetary Science Letters, 481, 387–394, https://doi.org/10.1016/j.epsl.2017.10.048, 2018.
Liu, J., Xiao, C., Ding, M., and Ren, J.: Variations in stable hydrogen and oxygen isotopes in atmospheric water vapor in the marine boundary layer across a wide latituderange, J. Environ. Sci., 26, 2266–2276, https://doi.org/10.1016/j.jes.2014.09.007, 2014.
Magh, R.-K., Gralher, B., Herbstritt, B., Kübert, A., Lim, H., Lundmark, T., and Marshall, J.: Technical note: Conservative storage of water vapour – practical in situ sampling of stable isotopes in tree stems, Hydrol. Earth Syst. Sci., 26, 3573–3587, https://doi.org/10.5194/hess-26-3573-2022, 2022.
Michener, R. and Lajtha, K.: Stable isotopes in ecology and environmental science, John Wiley & Sons, https://doi.org/10.1002/9780470691854, 2008.
Millar, C., Pratt, D., Schneider, D. J., and McDonnell, J. J.: A comparison of extraction systems for plant water stable isotope analysis, Rapid Communications in Mass Spectrometry, 32, 1031–1044, https://doi.org/10.1002/rcm.8136, 2018.
Moyer, E. J., Irion, F. W., Yung, Y. L., and Gunson, M. R.: ATMOS stratospheric deuterated water and implications for troposphere-stratosphere transport, Geophysical Research Letters, 23, 2385–2388, 10.1029/96gl01489, 1996.
Muccio, Z. and Jackson, G. P.: Isotope ratio mass spectrometry, Analyst, 134, 213–222, https://doi.org/10.1039/b808232d, 2009.
Rodgers, C. D. and Connor, B. J.: Intercomparison of remote sounding instruments, Journal of Geophysical Research: Atmospheres, 108, https://doi.org/10.1029/2002jd002299, 2003.
Rozmiarek, K. S., Vaughn, B. H., Jones, T. R., Morris, V., Skorski, W. B., Hughes, A. G., Elston, J., Wahl, S., Faber, A.-K., and Steen-Larsen, H. C.: An unmanned aerial vehicle sampling platform for atmospheric water vapor isotopes in polar environments, Atmos. Meas. Tech., 14, 7045–7067, https://doi.org/10.5194/amt-14-7045-2021, 2021.
Salmon, O. E., Welp, L. R., Baldwin, M. E., Hajny, K. D., Stirm, B. H., and Shepson, P. B.: Vertical profile observations of water vapor deuterium excess in the lower troposphere, Atmos. Chem. Phys., 19, 11525–11543, https://doi.org/10.5194/acp-19-11525-2019, 2019.
Schmidt, M., Maseyk, K., Lett, C., Biron, P., Richard, P., Bariac, T., and Seibt, U.: Concentration effects on laser-based δ18O and δ2H measurements and implications for the calibration of vapour measurements with liquid standards, Rapid Communications in Mass Spectrometry, 24, 3553–3561, https://doi.org/10.1002/rcm.4813, 2010.
Schneider, A., Borsdorff, T., aan de Brugh, J., Aemisegger, F., Feist, D. G., Kivi, R., Hase, F., Schneider, M., and Landgraf, J.: First data set of H2O HDO columns from the Tropospheric Monitoring Instrument (TROPOMI), Atmos. Meas. Tech., 13, 85–100, https://doi.org/10.5194/amt-13-85-2020, 2020.
Shi, X., Risi, C., Pu, T., Lacour, J. l., Kong, Y., Wang, K., He, Y., and Xia, D.: Variability of isotope composition of precipitation in the southeastern Tibetan Plateau from the synoptic to seasonal time scale, Journal of Geophysical Research: Atmospheres, 125, e2019JD031751, https://doi.org/10.1029/2019jd031751, 2020.
Smith, J. A., Ackerman, A. S., Jensen, E. J., and Toon, O. B.: Role of deep convection in establishing the isotopic composition of water vapor in the tropical transition layer, Geophysical Research Letters, 33, 6, https://doi.org/10.1029/2005gl024078, 2006.
Sprenger, M., Herbstritt, B., and Weiler, M.: Established methods and new opportunities for pore water stable isotope analysis, Hydrological Processes, 29, 5174–5192, https://doi.org/10.1002/hyp.10643, 2015.
Steen-Larsen, H. C., Masson-Delmotte, V., Sjolte, J., Johnsen, S. J., Vinther, B. M., Bréon, F. M., Clausen, H. B., Dahl-Jensen, D., Falourd, S., and Fettweis, X.: Understanding the climatic signal in the water stable isotope records from the NEEM shallow firn/ice cores in northwest Greenland, Journal of Geophysical Research Atmospheres, 116, 161–165, https://doi.org/10.1029/2010jd014311, 2011.
Wang, D.: wangdishi/Correcting-for-water-vapor-diffusion_AMT_F: AMT (AMT), Zenodo [code], https://doi.org/10.5281/zenodo.17790553, 2025.
Wassenaar, L., Hendry, M., Chostner, V., and Lis, G.: High resolution pore water δ2H and δ18O measurements by H2O (liquid)–H2O (vapor) equilibration laser spectroscopy, Environmental Science & Technology, 42, 9262–9267, https://doi.org/10.1021/es802065s, 2008.
Webster, C. R. and Heymsfield, A. J.: Water isotope ratios D/H, O-18/O-16, O-17/O-16 in and out of clouds map dehydration pathways, Science, 302, 1742–1745, https://doi.org/10.1126/science.1089496, 2003.
West, A. G., Goldsmith, G. R., Brooks, P. D., and Dawson, T. E.: Discrepancies between isotope ratio infrared spectroscopy and isotope ratio mass spectrometry for the stable isotope analysis of plant and soil waters, Rapid Communications in Mass Spectrometry, 24, 1948–1954, https://doi.org/10.1002/rcm.4597, 2010.
West, J. B., Bowen, G. J., Dawson, T. E., and Tu, K. P.: Isoscapes: understanding movement, pattern, and process on Earth through isotope mapping, Springer, https://doi.org/10.1007/s10933-011-9553-6, 2009.
Worden, J., Bowman, K., Noone, D., Beer, R., Clough, S., Eldering, A., Fisher, B., Goldman, A., Gunson, M., Herman, R., Kulawik, S. S., Lampel, M., Luo, M., Osterman, G., Rinsland, C., Rodgers, C., Sander, S., Shephard, M., and Worden, H.: Tropospheric emission spectrometer observations of the tropospheric HDO/H2O ratio: Estimation approach and characterization, Journal of Geophysical Research-Atmospheres, 111, https://doi.org/10.1029/2005jd006606, 2006.
Worden, J. R., Kulawik, S. S., Fu, D., Payne, V. H., Lipton, A. E., Polonsky, I., He, Y., Cady-Pereira, K., Moncet, J.-L., Herman, R. L., Irion, F. W., and Bowman, K. W.: Characterization and evaluation of AIRS-based estimates of the deuterium content of water vapor, Atmos. Meas. Tech., 12, 2331–2339, https://doi.org/10.5194/amt-12-2331-2019, 2019.
Yu, W., Tian, L., Ma, Y., Xu, B., and Qu, D.: Simultaneous monitoring of stable oxygen isotope composition in water vapour and precipitation over the central Tibetan Plateau, Atmos. Chem. Phys., 15, 10251–10262, https://doi.org/10.5194/acp-15-10251-2015, 2015.